Highlights
– A 60‑day, double‑blind RCT (n=60) tested a combined supplement containing riceberry anthocyanin (≈0.28 g/day) + inulin (Jerusalem artichoke) and rice bran (≈1.26 g/day each; total dose ≈2.8 g/day) vs. maltodextrin placebo.
– The supplement group showed statistically and clinically meaningful reductions in fasting plasma glucose (mean fall ≈45 mg/dL; interaction p=0.03), HbA1c (−0.7% absolute; interaction p=0.002) and LDL‑C (−14 mg/dL; interaction p=0.02). No safety signals were observed.
– Markers of oxidative stress (MDA), vitamin C, hsCRP and cardiorespiratory fitness did not change significantly; mechanisms remain speculative and require mechanistic studies.
Study background and disease burden
Type 2 diabetes mellitus (T2DM) is a leading global cause of morbidity and cardiometabolic disease. Even with pharmacotherapy, residual hyperglycaemia and dyslipidaemia contribute to cardiovascular risk. Nutraceutical and dietary adjuncts that are safe, inexpensive and scalable—especially those that modulate post‑absorptive glucose and lipid metabolism or gut microbiota—are attractive as complementary therapies.[1,4]
Anthocyanins (plant flavonoids present in pigmented grains and berries) have antioxidant and putative glucose‑lowering effects. Separately, fermentable soluble fibres such as inulin and rice bran modify gut microbiota, increase short‑chain fatty acids, and have been associated with improved glycaemia and lipids in some trials. Prior evidence largely evaluates single ingredients; combination (synbiotic‑style) products could plausibly have additive or synergistic benefits but have limited clinical trial data in T2DM.[9,17]
Study design
This was a randomized, double‑blind, placebo‑controlled trial conducted in Khon Kaen, Thailand. Key features:
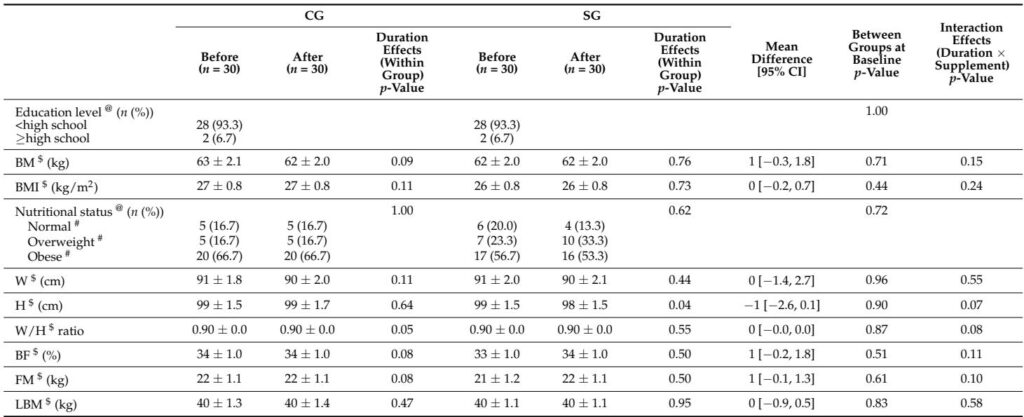
– Population: 60 adults with physician‑diagnosed T2DM (mostly female; 28F/2M per arm), on stable oral hypoglycaemic therapy and stable lipid/antihypertensive medications.
– Intervention: multi‑ingredient capsule blend (total ≈2.8 g/day delivered as 8 capsules/day of 350 mg each) containing riceberry anthocyanin (≈0.28 g/day) plus soluble fibres from Jerusalem artichoke (inulin) and white rice bran (≈1.26 g/day each). Placebo was maltodextrin.
– Duration: 60 days.
– Primary/secondary endpoints: fasting plasma glucose (FPG), HbA1c, lipids (LDL‑C, TC, TG, HDL‑C), insulin/HOMA‑IR, markers of oxidative stress (MDA), antioxidant status (vitamin C), inflammation (hsCRP, WBC), renal/liver safety (creatinine, eGFR, SGPT), and functional capacity (6‑minute walk test to estimate VO2peak).
– Analysis: ANCOVA for normally distributed outcomes, nonparametric tests for skewed variables; modified intention‑to‑treat.
Key findings
Population and adherence
– Sixty participants randomized 1:1 (30 per group); high adherence (≈97.8% in supplement group). No intervention‑related adverse events reported. Baseline characteristics and diet/physical activity remained balanced.
Glycaemic control
– Fasting plasma glucose: supplement group mean fell from 230.1 to 187.8 mg/dL (within‑group p=0.01); control 217.9 → 220.7 mg/dL (ns). ANCOVA interaction (duration × treatment) p=0.03. Mean adjusted difference ≈ −45 mg/dL (95% CI −76.1, −4.6) comparing change vs placebo.
– HbA1c: supplement group 8.6% → 7.9% (within‑group p=0.004); control 8.6% → 8.8% (ns). Interaction p=0.002. Absolute HbA1c reduction ≈0.7% (95% CI −1.5, −0.3).
– Insulin and HOMA‑IR: no significant within‑ or between‑group changes were observed over 60 days.
Lipid profile
– LDL‑C: supplement group decreased from 96 to 82 mg/dL (within‑group p=0.006); control unchanged (101 → 102 mg/dL). Interaction p=0.02. Absolute mean difference ≈ −15 mg/dL (95% CI −29.1, −2.8).
– Total cholesterol was reduced in the supplement group (174 → 160 mg/dL; within‑group p=0.002) but interaction did not reach statistical significance. TG and HDL‑C did not change meaningfully.
Inflammation, oxidative stress, antioxidant status
– Plasma malondialdehyde (MDA), vitamin C, hsCRP and most leukocyte subsets did not show statistically significant changes. A trend toward lower hsCRP and WBC in the supplement group (p≈0.07) was noted but not definitive.
Renal and liver safety
– No adverse changes in serum creatinine or SGPT. eGFR improved modestly in the supplement group (90.8 → 95.1 mL/min/1.73 m2; within‑group p=0.01); not statistically significant between groups.
Functional capacity
– No significant effect on 6‑minute walk distance or estimated VO2peak.
Interpretation of effect sizes
– The HbA1c reduction of ≈0.7% over 60 days is clinically relevant in the T2DM setting and comparable to some dietary intervention effects reported in short trials.[13,45] LDL‑C reduction of ≈14% is also potentially meaningful for cardiovascular risk when sustained.
Expert commentary and mechanistic plausibility
Mechanisms that could plausibly account for the observed glycaemic and lipid effects include:
– Direct intestinal enzyme inhibition: anthocyanins can inhibit α‑glucosidase and α‑amylase, slowing carbohydrate absorption and postprandial glycaemia.[54]
– Gut microbiota and SCFA production: inulin and rice bran are fermentable substrates that increase short‑chain fatty acids (acetate, propionate, butyrate), which modulate hepatic gluconeogenesis, incretin release and lipid synthesis.[55,58]
– Insulin signalling and peripheral glucose uptake: rice bran phenolics may enhance IRS1/AKT/GLUT4 signalling in muscle (preclinical evidence).[57]
– Lipid metabolism: inulin fermentation and anthocyanin‑mediated effects (including CETP inhibition and increased cholesterol efflux) may combine to lower LDL‑C.[60]
Limitations and generalisability
– Short duration (60 days): HbA1c reflects ~8–12 weeks of glycaemia; a 60‑day window produces partial HbA1c change and longer follow‑up is needed to demonstrate sustainable benefit.
– Small sample and sex distribution: the study enrolled mostly women (56/60 initially); results may not generalize to men or other ethnicities.
– Combination product: because the intervention contained multiple active ingredients, the trial cannot attribute effects to anthocyanin versus inulin versus rice bran or to synergy between them. A factorial design would be needed to isolate components.
– Mechanistic data missing: the trial did not measure gut microbiota composition, SCFA, or incretins (e.g., GIP/GLP‑1), which would strengthen mechanistic claims.
– Concomitant medications: participants remained on stable glucose‑lowering and lipid‑modifying therapies; interactions appear absent but were not formally tested across medication strata.
Comparison with prior evidence
– The magnitude of glycaemic and lipid improvement is consistent with prior smaller or single‑ingredient trials of inulin or anthocyanin (e.g., Qureshi 2002 for rice bran; Li 2015 for purified anthocyanin), but this trial used relatively modest anthocyanin and fibre doses and achieved clinically meaningful effects within 60 days.[13,45]
Clinical implications and next steps
For clinicians: the study provides randomized evidence that a combined riceberry anthocyanin + inulin + rice bran supplement may improve short‑term glycaemia and LDL‑C as an adjunct to standard therapy in motivated patients with T2DM. High adherence and absence of safety signals are encouraging.
Caveats for practice
– Do not replace proven glucose‑lowering or lipid‑lowering medications with nutraceuticals.
– Discuss supplement use with patients to avoid unknown interactions and ensure quality control.
Recommended research priorities
– Larger, longer randomized trials (≥6 months) with diverse populations and stratification by baseline therapy.
– Factorial or component trials to dissociate anthocyanin vs inulin vs rice bran effects.
– Mechanistic studies measuring gut microbiome, SCFA, incretin hormones (GIP/GLP‑1), postprandial glycaemia, and insulin sensitivity (e.g., clamp studies).
– Dose‑finding studies to establish optimal anthocyanin and inulin content for efficacy and tolerability.
Conclusions
This well‑conducted 60‑day randomized, double‑blind trial reports that a combined anthocyanin (riceberry) plus prebiotic soluble fibre (inulin from Jerusalem artichoke and rice bran) supplement produced clinically meaningful reductions in fasting glucose, HbA1c and LDL‑C versus placebo in people with T2DM, with good adherence and no adverse events. Oxidative stress and inflammatory biomarkers were unchanged. The findings support further investigation in larger, longer trials and suggest that this multi‑ingredient supplement could be considered as a complementary adjunct under clinical supervision, pending confirmatory data.
References
Teparak C, Uriyapongson J, Phoemsapthawee J, Tunkamnerdthai O, Aneknan P, Tong-Un T, Panthongviriyakul C, Leelayuwat N, Alkhatib A. Diabetes Therapeutics of Prebiotic Soluble Dietary Fibre and Antioxidant Anthocyanin Supplement in Patients with Type 2 Diabetes: Randomised Placebo-Controlled Clinical Trial. Nutrients. 2025 Mar 21;17(7):1098. doi: 10.3390/nu17071098 IF: 5.0 Q1 . PMID: 40218856 IF: 5.0 Q1 ; PMCID: PMC11990404 IF: 5.0 Q1 .