Highlights
- Anrikefon, a selective peripherally restricted kappa opioid receptor agonist, significantly reduces itch intensity in CKD-associated pruritus among haemodialysis patients.
- 37% of patients treated with anrikefon achieved at least a 4-point reduction on the WI-NRS at 12 weeks, compared with 15% in placebo (P<0.001).
- Improvements in itch-related quality of life were evidenced by significant reductions in 5-D itch and Skindex-10 scales, sustained through 40 weeks during open-label extension.
- The safety profile was favorable, with mild to moderate dizziness being the predominant adverse event, without clinical sequelae.
Background
Chronic kidney disease-associated pruritus (CKD-aP) is a prevalent, distressing symptom in patients undergoing haemodialysis, affecting up to 40% of this population. CKD-aP substantially impairs quality of life, sleep, and overall wellbeing and has been linked with increased morbidity and mortality. Despite its clinical impact, treatment options remain limited and frequently unsatisfactory.
Kappa opioid receptor (KOR) agonists have emerged as a promising therapeutic class owing to their regulation of sensory nerve signaling implicated in pruritus. However, centrally acting KOR agonists can produce undesirable CNS effects. Anrikefon (formerly HSK21542) is a novel selective peripherally restricted KOR agonist designed to reduce pruritus intensity without central nervous system penetration, thereby potentially minimizing adverse CNS effects.
Key Content
Study Design and Population
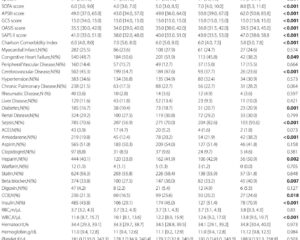
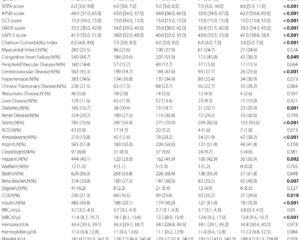
Between June 2022 and June 2024, a multicentre, double-blind, randomized placebo-controlled phase 3 trial was conducted across 50 centres in China enrolling adult haemodialysis patients with moderate to severe CKD-aP. A total of 652 patients were screened, and 545 were randomized 1:1 to receive intravenous anrikefon (0.3 µg/kg) or placebo thrice weekly for 12 weeks, followed by an optional 40-week open-label extension with anrikefon.
Primary and Secondary Outcomes
The primary endpoint was the proportion of patients achieving a ≥4 point reduction in weekly mean 24-hour Worst Itching Intensity Numerical Rating Scale (WI-NRS) at week 12 versus baseline. Secondary endpoints included proportions achieving ≥3 point WI-NRS reduction, and improvements in itch-related quality of life assessed by the Skindex-10 and 5-D itch scales. Safety outcomes included adverse event incidence and severity throughout treatment.
Efficacy Results
Among 275 patients treated with anrikefon, 37% met the primary endpoint versus 15% in the placebo group (P<0.001). For the secondary endpoint (≥3 point WI-NRS reduction), 51% were responders in the anrikefon group compared with 24% in placebo (P<0.001). Anrikefon recipients also exhibited significantly greater mean reductions in 5-D itch scale (-5.3 vs -3.1, P<0.001) and Skindex-10 score (-15.2 vs -9.3, P<0.001), indicating meaningful improvement in itch-related quality of life.
The sustained improvements were confirmed during the 40-week open-label extension, with persistent quality of life benefits using the 5-D itch scale.
Safety Profile
The safety analysis showed that anrikefon was generally well tolerated. Mild to moderate dizziness occurred more frequently in the anrikefon group than placebo but had no major clinical consequences. Other adverse events were similar between groups, and no significant central nervous system adverse effects were reported, consistent with the drug’s peripheral restriction.
Expert Commentary
This pivotal phase 3 trial provides robust evidence supporting anrikefon as an effective and safe treatment for moderate to severe CKD-aP in haemodialysis patients—an area with significant unmet clinical need. The peripheral selectivity of anrikefon avoids central nervous system-related side effects that have limited the use of other KOR agonists, making it a promising therapeutic agent.
The magnitude of itch intensity reduction (≥4 points in 37% of patients) meets clinically meaningful thresholds and is supported by parallel improvements in validated quality of life scales. The longer-term data from the extension phase are encouraging, suggesting that benefits are sustainable without progressive safety concerns.
However, despite statistical significance, less than half of patients achieved the primary endpoint, highlighting remaining challenges in CKD-aP management and the heterogeneity of patients’ response. Further studies exploring combinational therapies, biomarkers predicting response, and head-to-head comparisons with other antipruritic agents would be valuable.
The study’s large multicentre design and rigorous double-blind randomization confer high validity and applicability in real-world haemodialysis populations, particularly within Asian cohorts, although extrapolation to other ethnic groups warrants further evaluation.
Conclusion
Anrikefon represents a significant advancement for the management of CKD-associated pruritus in haemodialysis patients, offering an effective, well-tolerated therapy that improves both symptom burden and quality of life. Pending regulatory approval, anrikefon could fill a substantial therapeutic gap, improving patient outcomes in this difficult-to-treat population. Future research should focus on broader population validation, long-term safety, and integration into CKD-aP clinical guidelines.
References
- Liu BC, Li ZL, Zhang P, Zhong AM, Bai YL, Xu Y, Gao BH, Li YL, Wang Y, Zhou LH, Yao L, Wang JX, Yan R, Wang L, Liao B, Xie DQ, Yi XM, Guan TJ, Wang CL, Li GS, Li FQ, Chen JH; Anrikefon-302 study collaborator group. Efficacy and safety of anrikefon in patients with pruritus undergoing haemodialysis: multicentre, double blind, randomised placebo controlled phase 3 trial. BMJ. 2025 Aug 19;390:e085208. doi:10.1136/bmj-2025-085208. PMID: 40829896.