Introduction
Atrial fibrillation (AF) is the most prevalent cardiac arrhythmia worldwide, associated with significant morbidity, including stroke, heart failure, and increased mortality. AF’s burden intensifies in critically ill patients admitted to intensive care units (ICUs), where its incidence reaches approximately 15.6%, often complicating the clinical course and worsening prognosis. Despite its frequency, prognostic biomarkers tailored for AF in critical illness remain insufficiently characterized.
Insulin resistance (IR), a hallmark of metabolic syndrome and type 2 diabetes, is increasingly recognized as a contributor to cardiovascular disease and arrhythmogenesis. The triglyceride-glucose (TyG) index, derived simply from fasting triglyceride and glucose measurements, has emerged as a validated surrogate marker for IR. Prior studies link elevated TyG index with adverse cardiovascular outcomes and poor prognosis in AF; however, most rely on single time-point measurements, limiting their prognostic utility, especially in ICU patients who experience physiological stress-induced metabolic fluctuations.
Dynamic assessment of the TyG index over time, namely its trajectory during ICU stay, may better capture metabolic instability and forecast patient outcomes. This retrospective cohort study utilizes the Medical Information Mart for Intensive Care (MIMIC)-IV database to investigate the association between TyG index trajectories and all-cause mortality among critically ill AF patients.
Study Design and Methods
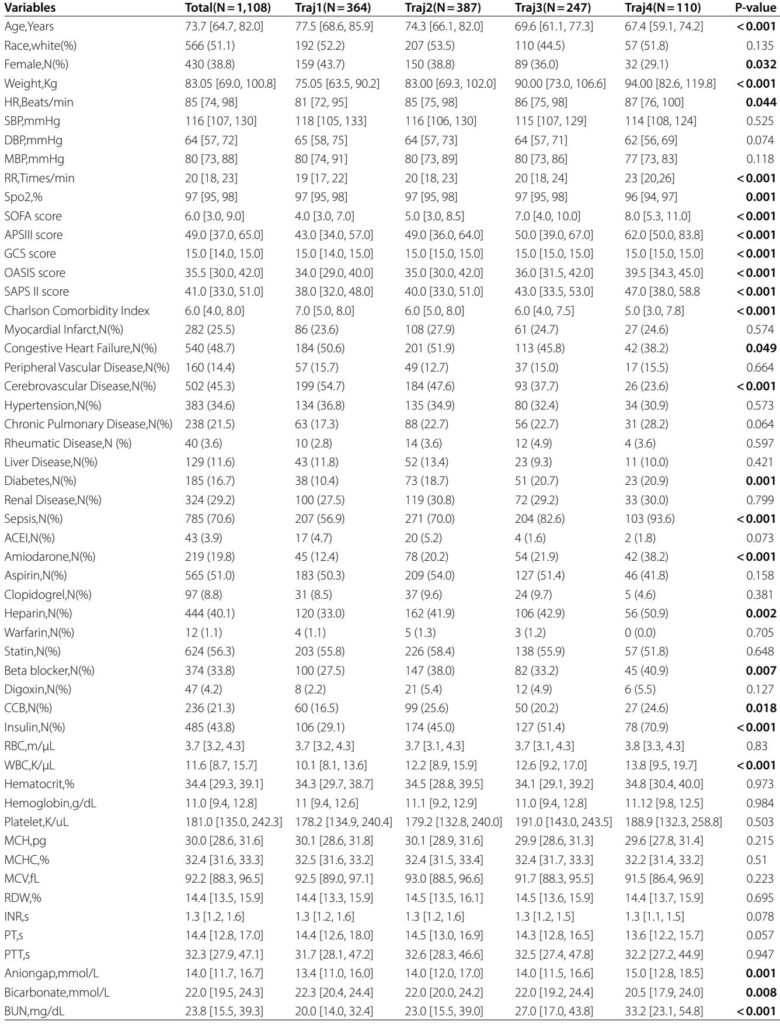
This retrospective cohort study enrolled patients from the MIMIC-IV database (version 3.1) diagnosed with AF on ICU admission between 2008 and 2022. Inclusion criteria were age ≥18 years, first ICU admission with a diagnosis of AF, an ICU length of stay exceeding 72 hours, and at least three paired measurements of blood glucose and triglyceride within 24-hour windows during ICU stay.
Patients with cancer or insufficient biochemical data were excluded to ensure data integrity. The TyG index was calculated as ln(fasting triglycerides [mg/dL] × fasting glucose [mg/dL] ÷ 2). Group-based trajectory modeling (GBTM) identified distinct patterns of TyG index changes during ICU stay, selecting a 4-class model based on Bayesian and Akaike information criteria, posterior probabilities, and clinical interpretability.
Primary outcomes included 30-day and 365-day all-cause mortality; secondary outcomes covered 90-day and 180-day mortality. Survival differences were assessed using Kaplan–Meier curves and log-rank tests. Cox proportional hazards models adjusted for demographic, clinical, laboratory, and treatment covariates estimated hazard ratios (HRs) for mortality across TyG trajectory groups. Restricted cubic spline analysis explored potential nonlinear associations between TyG levels and mortality.
Key Findings
In total, 1,108 critically ill patients with AF were classified into four TyG index trajectory groups:
- traj1 (stable-low): TyG index persistently low (31.2%)
- traj2 (slowly ascending): gradual moderate increase (35.7%)
- traj3 (ascending then descending): moderate-high fluctuations (23.1%)
- traj4 (fluctuating-high): high and unstable levels (10.0%)
Patients in the traj4 group demonstrated significantly higher all-cause mortality at all assessed time points: 30-day (40.0%), 90-day (49.1%), 180-day (50.0%), and 365-day (58.2%) compared to the other groups (p < 0.05 for all comparisons).
Adjusted Cox models revealed that traj4 patients had approximately 1.4 to 1.7 fold increased risk of mortality relative to the stable-low group, after controlling for confounders including age, sex, comorbidities, and treatments. No significant mortality differences were observed for traj2 and traj3 compared to traj1.
Restricted cubic spline analyses indicated a linear dose-response relationship between increasing TyG index levels and higher mortality, underscoring the relevance of dynamic metabolic status over static measurement.
Subgroup analyses confirmed that the association of fluctuating high TyG trajectory with increased mortality persisted across age strata, sex, race, comorbidities, and medication subgroups, signifying robust predictive capacity.
Expert Commentary
This study pioneers the application of trajectory modeling of the TyG index to critically ill AF patients, offering important mechanistic and clinical insights. IR contributes to structural and electrical remodeling of the atrium via myocardial fibrosis, inflammation, and autonomic dysfunction, thereby fostering AF initiation and progression. The fluctuations in TyG index likely reflect metabolic instability and heightened oxidative stress, exacerbating cardiac fibrosis and arrhythmogenic substrate.
The stable or mildly increasing TyG levels were not independently associated with mortality, highlighting that dynamic metabolic swings at elevated levels critically influence prognosis. Clinically, these findings point to the potential utility of serial TyG monitoring in ICU settings to identify high-risk AF patients who might benefit from targeted metabolic interventions.
Several limitations warrant consideration: this retrospective, single-center study relies on intermittent measurements without guaranteed fasting states, though glucose variations predominantly drive TyG changes. Residual confounding despite multivariable adjustment cannot be excluded. External validation in multicenter cohorts and prospective studies is essential to generalize findings and explore interventional strategies.
Conclusion
In critically ill patients with atrial fibrillation, trajectories of the triglyceride-glucose index that fluctuate at high levels are independently associated with increased short- and long-term all-cause mortality. Serial assessment of TyG index trajectories provides a robust, accessible prognostic biomarker for risk stratification in ICU AF patients, with potential to guide personalized metabolic management and improve outcomes. Future prospective and interventional studies are warranted to confirm causality and evaluate therapeutic impact.