Background
Alzheimer’s disease (AD), a progressive neurodegenerative disorder, manifests in three stages: pre-clinical, prodromal (mild cognitive impairment), and clinical dementia. Emerging evidence indicates cerebrovascular changes, including reduced cerebral blood flow, precede symptomatic AD by up to two decades. Hypertension, a major global health concern affecting a third of adults, may interact bidirectionally with AD pathogenesis. Although observational studies suggest midlife hypertension elevates AD risk, later-life high blood pressure associates inconsistently with dementia outcomes. Prior randomized controlled trials (RCTs) investigating antihypertensive therapy’s influence on AD risk have yielded inconclusive evidence due to methodological constraints and insufficient power.
This complexity stems partly from the difficulty in measuring pre-clinical AD and disentangling confounding and reverse causality in observational research. Consequently, rigorous causal inference methods such as instrumental variable analyses—including Mendelian randomisation (MR)—have been employed to probe the directionality between AD and blood pressure (BP). Notably, limited research has focused on the impact of pre-clinical AD on systemic BP. Addressing this gap is crucial for understanding potential pathophysiological mechanisms linking brain pathology and peripheral vascular regulation.
Study Design and Methods
This investigation utilized data from the UK Biobank, encompassing over 500,000 adults aged 40–69 years, after excluding individuals with prevalent or incident dementia within five years of enrollment to minimize reverse causality. Two complementary instrumental variables were employed to proxy risk of pre-clinical AD:
- A parental dementia instrument score, derived from participant reports of parental dementia status, adjusted for parental age to gauge diagnostic certainty, thereby capturing both genetic and environmental influences.
- A participant genetic instrument score, constructed from 32 genome-wide significant single nucleotide polymorphisms (SNPs) linked to AD risk from an independent genome-wide association study (GWAS), thus serving as a genetic liability proxy unaffected by confounding.
Analyses restricted to European ancestry participants mitigated confounding by population stratification. Outcome measures included systolic BP (SBP), diastolic BP (DBP), and hypertension status, with adjustments for antihypertensive medication effects. Confounders such as age, sex, body mass index, lifestyle factors, and socioeconomic status were accounted for primarily in parental dementia instrument analyses due to plausible confounding pathways.
Key Findings
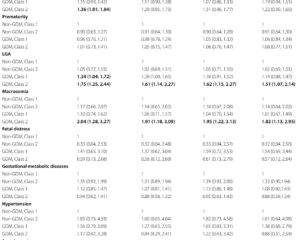
Both instrumental variables indicated a statistically significant association between increased risk of pre-clinical AD and elevated SBP: the parental dementia instrument score corresponded to a 0.12 mmHg increase in mean SBP per standard deviation (95% confidence interval [CI]: 0.06–0.17, p < 0.0001), while the participant genetic instrument score showed a 0.07 mmHg elevation (95% CI: 0.00–0.13, p = 0.037). No consistent associations were observed for DBP.
Moreover, the parental dementia instrument score was linked to a modest increase in hypertension odds (odds ratio [OR] 1.021, 95% CI: 1.014–1.028, p < 0.0001), with a weaker non-significant trend for the genetic instrument. Sensitivity analyses, including leave-one-out SNP testing and adjustment for additional metabolic markers, confirmed the robustness of these estimates.
Importantly, adjusting for age reversed the direction of association for the parental dementia instrument from negative to positive, emphasizing the critical role of confounding correction. The genetic instrument’s stability across adjustments further enhanced confidence in the causal inference.
Expert Commentary
This study corroborates a novel aspect of the hypothesized bi-directional relationship between blood pressure and Alzheimer’s disease, specifically supporting that pre-clinical AD leads to increases in systemic SBP. Physiologically, cerebral hypoperfusion, an early hallmark of AD, may trigger compensatory elevation in peripheral BP to maintain adequate cerebral perfusion. Animal models aligning with these findings demonstrate amyloid-beta–induced hypertension mediated by cerebral vasoconstrictors such as endothelin-1.
Clinically, this underscores the complexity of vascular contributions to cognitive decline and may explain inconsistencies in prior observational and intervention studies assessing the impact of BP manipulation on dementia risk. The distinction in SBP and DBP effects aligns with established vascular ageing patterns—arterial stiffness elevating SBP while DBP declines or remains unchanged.
Nevertheless, several limitations warrant cautious interpretation. The parental dementia instrument may be confounded by shared lifestyle and socioeconomic factors despite adjustment, while genetic instruments remain susceptible to horizontal pleiotropy. Exclusion of dementia cases within five years reduces but does not eliminate misclassification of pre-clinical states. Future integration of cerebral blood flow imaging and biomarker data could refine causal pathways.
Conclusion
Leveraging two independent instrumental variables, this analysis provides compelling evidence that pre-clinical Alzheimer’s disease causally elevates systolic blood pressure prior to clinical dementia onset. These findings enhance understanding of AD’s early pathophysiological effects on systemic vascular regulation and highlight the potential for blood pressure management tailored to dementia risk profiles.
Continued research integrating neuroimaging, fluid biomarkers (e.g., plasma phospho-Tau isoforms), and longitudinal BP monitoring is essential to elucidate the mechanistic underpinnings and therapeutic implications. Optimizing antihypertensive strategies to preserve cerebral perfusion without exacerbating vascular injury may offer avenues to delay or prevent overt cognitive decline.
Ultimately, recognition of elevated SBP as a potential biomarker of pre-clinical AD risk might inform earlier diagnostic screening and personalized intervention efforts, contributing to improved clinical outcomes in aging populations.