Highlights
- Inhaled molgramostim significantly improves diffusing capacity of the lungs for carbon monoxide (DLCO) in patients with autoimmune pulmonary alveolar proteinosis (aPAP) compared to placebo.
- This phase 3 trial demonstrates sustained benefit over 48 weeks on pulmonary function and health-related quality of life.
- Molgramostim inhalation shows a safety profile comparable to placebo, supporting its potential as a non-invasive therapy for aPAP.
- Earlier phase trials and other studies corroborate the role of inhaled GM-CSF in restoring alveolar macrophage function impaired by anti-GM-CSF autoantibodies.
Background
Autoimmune pulmonary alveolar proteinosis (aPAP) is an ultra-rare pulmonary disorder characterized by the accumulation of surfactant within alveoli, leading to impaired gas exchange and hypoxemia. The underlying pathophysiology involves autoantibodies against granulocyte–macrophage colony-stimulating factor (GM-CSF), a cytokine essential for alveolar macrophage maturation and surfactant clearance. Standard treatments have traditionally involved invasive procedures such as whole-lung lavage, and until recently, targeted pharmacologic options were limited. Recombinant human GM-CSF formulations, including molgramostim (inhaled) and sargramostim, have emerged as promising therapeutic candidates to restore macrophage-mediated surfactant clearance. This review synthesizes the latest phase 3 clinical evidence and prior studies assessing inhaled molgramostim’s efficacy and safety in aPAP.
Key Content
Chronological Development of Evidence on Inhaled GM-CSF in aPAP
The concept of inhaled GM-CSF therapy in aPAP was first clinically explored in early-phase studies. A 2019 randomized, placebo-controlled phase 2 trial of inhaled sargramostim demonstrated a modest but significant improvement in the alveolar-arterial oxygen gradient and lung density on computed tomography after 24 weeks of intermittent dosing, primarily in mild to moderate disease. However, clinical benefits such as symptom relief were limited, and the trial excluded patients with severe hypoxemia due to safety concerns.
Further advancing the field, a 2020 double-blind, placebo-controlled trial examined continuous versus intermittent inhaled molgramostim at 300 µg daily for 24 weeks, enrolling 138 patients with aPAP. Continuous daily dosing showed statistically significant improvements in oxygenation (measured via A-aDo2) and quality-of-life metrics compared to both intermittent dosing and placebo, with tolerable safety profiles. This study established the rationale for dose and frequency selection in subsequent trials.
Phase 3 Trial: IMPALA-2 Study of Inhaled Molgramostim
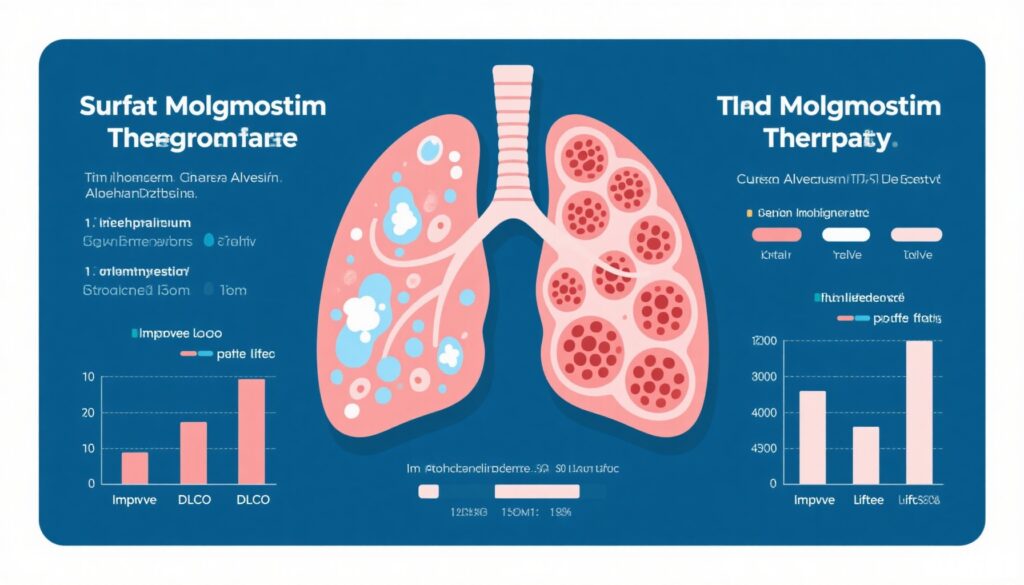
The recent phase 3, randomized, double-blind, placebo-controlled IMPALA-2 trial (NCT04544293) enrolled 164 patients with aPAP, randomized 1:1 to receive 300 µg of inhaled molgramostim or placebo once daily for 48 weeks. The primary endpoint was the change from baseline to 24 weeks in the diffusing capacity of the lungs for carbon monoxide (DLCO), adjusted for hemoglobin, expressed as a percent predicted. Secondary endpoints included DLCO at 48 weeks, changes in the St. George’s Respiratory Questionnaire total and activity scores (SGRQ-T and SGRQ-A), and exercise capacity.
Results revealed that molgramostim treatment led to a mean DLCO improvement of 9.8 percentage points at 24 weeks versus 3.8 percentage points for placebo (treatment difference 6.0; 95% CI, 2.5–9.4; P<0.001). At 48 weeks, the improvement further increased to 11.6 points versus 4.7 points (P<0.001). Quality of life measured by the SGRQ total score showed a significant decrease (improvement) in the molgramostim group at 24 weeks (−11.5 vs. −4.9 points; P=0.007), while activity scores did not differ significantly.
Safety outcomes were comparable between groups, with similar incidences of adverse and serious adverse events, supporting the favorable tolerability of inhaled molgramostim.
Mechanistic and Translational Insights
aPAP pathogenesis centers on autoantibodies neutralizing GM-CSF, disrupting alveolar macrophage maturation and function, thus impeding surfactant clearance. Molgramostim, by delivering recombinant human GM-CSF directly to the alveoli via inhalation, bypasses systemic circulation and targets alveolar macrophages to restore their surfactant catabolic activity.
Previous studies observed increased alveolar macrophage numbers and improved macrophage function following inhaled GM-CSF therapy, correlating with improved gas exchange. The phase 3 data reinforce that consistent daily dosing optimizes this biological effect, providing sustained pulmonary functional and symptomatic improvement.
Safety and Adverse Events
The phase 3 IMPALA-2 trial showed that inhaled molgramostim was well tolerated with no significant increase in adverse events compared to placebo. Earlier trials noted a slightly higher incidence of chest pain with continuous dosing, but overall safety was acceptable. Given the chronic nature of aPAP, the addition of an inhaled pharmacotherapy with a benign safety profile represents an important therapeutic advance over invasive treatments.
Expert Commentary
The phase 3 trial of inhaled molgramostim represents a milestone in the treatment of aPAP by demonstrating clinically meaningful improvements in gas exchange and quality of life in a rigorous, placebo-controlled setting. The sustained treatment benefits at 48 weeks underscore the importance of continued therapy to maintain pulmonary function gains.
Despite improvements in DLCO and patient-reported outcomes, the lack of significant change in activity scores highlights ongoing challenges in fully restoring exercise capacity, possibly due to comorbidities or structural lung changes. Ongoing investigation into earlier treatment initiation, combination approaches, and biomarkers of response is warranted.
Guideline incorporation of inhaled molgramostim could provide a non-invasive, pharmacologic alternative to whole-lung lavage, reducing healthcare burden and procedural risks. Limitations include the moderate sample size, and longer-term safety and efficacy data are desirable to confirm durability and safety profile.
Conclusion
Emerging evidence from phase 2 and phase 3 trials firmly establishes inhaled molgramostim as an effective and safe treatment option for autoimmune pulmonary alveolar proteinosis. By augmenting pulmonary GM-CSF signaling, this therapy improves alveolar macrophage function, enhances gas transfer, and improves health-related quality of life. These advances offer new hope for a rare disease with limited prior options and should inform updates to clinical practice guidelines.
References
- Schwartz DA, et al. Phase 3 Trial of Inhaled Molgramostim in Autoimmune Pulmonary Alveolar Proteinosis. N Engl J Med. 2024; DOI:10.1056/NEJMoa2410542.
- Tazawa R, et al. Inhaled Molgramostim Therapy in Autoimmune Pulmonary Alveolar Proteinosis. N Engl J Med. 2020 Oct 22;383(17):1635–1644. PMID: 32897035.
- Tazawa R, et al. Inhaled GM-CSF for Pulmonary Alveolar Proteinosis. N Engl J Med. 2019 Sep 5;381(10):923–932. PMID: 31483963.