Study Background and Disease Burden
Colorectal cancer (CRC) remains a leading cause of cancer-related morbidity and mortality worldwide, with a significant clinical need for improved therapeutic strategies, especially for patients refractory to immune checkpoint inhibitors (ICIs). The tumor microenvironment of CRC often exhibits immune suppression, limiting the effectiveness of immunotherapies such as PD-1 inhibitors. Emerging evidence underscores the impact of the gut microbiome on cancer progression and therapy response. Among gut-resident bacteria, Clostridium butyricum, a butyrate-producing probiotic, has attracted attention for its immunomodulatory potential. However, the precise molecular mechanisms underlying its antitumor effects have remained incompletely understood.
Study Design
A multidisciplinary research team led by Professor Jun Yu at The Chinese University of Hong Kong recently published a pivotal study in Cancer Cell elucidating the anticancer mechanism of a specific C. butyricum strain, Hkyucb11. The researchers isolated the bacterium from healthy adult fecal samples. Using in vitro CRC cell lines, patient-derived organoids, and multiple in vivo murine CRC models—including immunotherapy-resistant variants—they evaluated the impact of Hkyucb11 alone and combined with PD-1 blockade. They conducted tumor growth assays, immunophenotyping of tumor-infiltrating lymphocytes (TILs) via flow cytometry, cytokine profiling, and molecular interaction studies to identify bacterial and cancer cell surface proteins mediating the effect. Transcriptomic and signaling pathway analyses focused on immunosuppressive cytokines and canonical oncogenic pathways.
Key Findings
The research demonstrated that the Hkyucb11 strain selectively reduced colorectal cancer cell viability and induced apoptosis in vitro without harming normal colonic epithelial cells. This anticancer effect translated into significant tumor burden reduction in CRC mouse models treated with Hkyucb11, with no adverse effects on overall mouse health or organ function.
In the tumor microenvironment, Hkyucb11 treatment increased the proportion and activation of cytotoxic CD8+ T cells as evidenced by elevated IFN-γ, TNF-α, and granzyme B expression. Concurrently, infiltration by M2-type pro-tumoral macrophages was suppressed. These immune microenvironment changes mirror those observed in enhanced immunotherapy responses.
Critically, combining Hkyucb11 with PD-1 blockade markedly amplified antitumor efficacy compared to monotherapies, including in immune checkpoint inhibitor–resistant models. Depletion of CD8+ T cells abrogated Hkyucb11’s therapeutic effect, underscoring the indispensable role of CD8+ T cells in the probiotic’s antitumor mechanism.
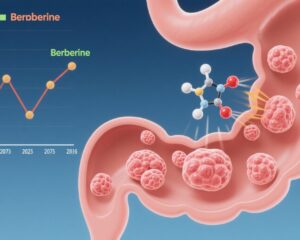
Mechanistically, the authors identified a direct interaction between C. butyricum surface protein secD and the colorectal cancer cell surface receptor glucose-regulated protein 78 (GRP78). GRP78 is selectively overexpressed on CRC cells but not normal colonic epithelium. Binding of secD to GRP78 diminished GRP78 levels on tumor cells and suppressed downstream PI3K-AKT-NF-κB signaling. This signaling inhibition reduced secretion of the immunosuppressive cytokine IL-6, thereby relieving T cell suppression and promoting T cell activation.
In addition to direct bacterial-cell contact effects, butyrate production by C. butyricum contributed to the antitumor response and partially enhanced immunotherapy outcomes.
The team validated these mechanistic insights across various humanized and syngeneic CRC mouse models, confirming broad translational relevance.
Expert Commentary
This study from The Chinese University of Hong Kong represents a landmark in microbiome-oncology research by illuminating a direct molecular interaction through which a probiotic modulates tumor immune evasion. The secD-GRP78 axis represents a novel targetable pathway that links bacterial colonization directly to attenuation of tumor-driven immune suppression.
These findings complement and extend previous reports from institutions such as the University of Zurich and City of Hope Comprehensive Cancer Center that indicated commensal Clostridiales strains or CBM588 probiotic formulations could enhance immunotherapy efficacy via CD8+ T cell modulation [1,2]. This current mechanistic elucidation provides a firm molecular basis for such clinical observations.
While this study robustly demonstrates safety and efficacy in preclinical models, clinical trials are needed to confirm benefits in human CRC patients, especially those with immunotherapy-resistant tumors. Given that GRP78 is implicated in diverse cancer signaling networks, potential off-target or resistance mechanisms warrant examination. Nevertheless, this research positions C. butyricum and its secD protein as promising adjuncts to immunotherapy in colorectal cancer management.
Conclusion
The discovery that Clostridium butyricum can directly interact with colorectal cancer cells via secD-GRP78 binding to inhibit PI3K-AKT-NF-κB signaling and reduce IL-6–mediated immune suppression furnishes a novel paradigm for probiotic-assisted cancer immunotherapy. By restoring CD8+ T cell antitumor immunity and enhancing PD-1 checkpoint blockade efficacy, this probiotic offers a potential new therapeutic avenue for colorectal cancer, including checkpoint inhibitor–resistant cases.
Future clinical translation of these findings may lead to effective microbiome-based adjunct therapies that improve outcomes and overcome current limitations of immunotherapy in CRC.
References
[1] Montalban-Arques A, et al. Commensal Clostridiales strains mediate effective anti-cancer immune response against solid tumors. Cell Host Microbe. 2021;29(10):1573-1588.e7.
[2] Dizman N, et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: a randomized phase 1 trial. Nat Med. 2022;28(4):704-712.
[3] Xie M, Yuan K, Zhang Y, et al. Tumor-resident probiotic Clostridium butyricum improves aPD-1 efficacy in colorectal cancer models by inhibiting IL-6-mediated immunosuppression. Cancer Cell. 2025; published online July 29.
[4] Dai Z, et al. Multi-cohort analysis of colorectal cancer metagenome identified altered bacteria across populations and universal bacterial markers. Microbiome. 2018;6(1):70.
[5] Araujo N, Hebbar N, Rangnekar VM. GRP78 is a targetable receptor on cancer and stromal cells. EBioMedicine. 2018;33:2-3.