Highlight
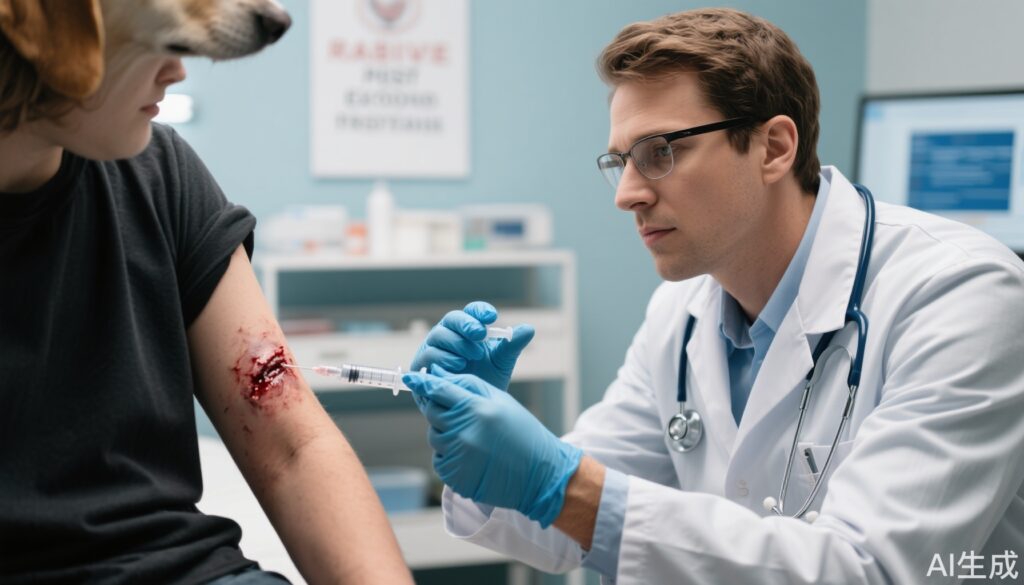
– Rabies monoclonal antibody (RmAb) combined with purified Vero cell rabies vaccine (PVRV) provides effective post-exposure prophylaxis (PEP) in category 3 exposures.
– The phase 4, open-label randomized trial in India showed no development of rabies in 3994 treated patients over one year.
– The safety profile of RmAb plus vaccine was favorable with only mild, transient adverse events, and serious adverse events were unrelated to treatment.
– Immunogenicity was robust with sustained rabies virus neutralizing antibody levels at day 14 and beyond.
Study Background and Disease Burden
Rabies is a universally fatal viral zoonosis once clinical symptoms develop. Globally significant, especially in low- and middle-income countries such as India, rabies causes thousands of deaths yearly. Post-exposure prophylaxis (PEP), including passive immunization with rabies immunoglobulins and active vaccination, is crucial after potential rabies exposure to prevent disease onset. Traditionally, equine rabies immunoglobulin (ERIG) has been used for passive immunity but has limitations including availability, batch variability, and risk of adverse reactions. The development and approval of rabies monoclonal antibodies (RmAb), such as Rabishield, introduce a promising alternative for passive prophylaxis with potentially better safety, consistent potency, and scalability. This study aims to evaluate the long-term safety, immunogenicity, and efficacy of a PEP regimen combining RmAb and purified Vero cell rabies vaccine (PVRV) compared to the ERIG plus PVRV standard in WHO category 3 suspected rabies exposures.
Study Design
This phase 4, open-label, randomized, active-controlled trial was conducted across 15 tertiary care hospitals in India between August 2019 and March 2022. Eligible participants were aged 2 years or older with WHO category 3 potential rabies exposure by a suspected rabid animal. Inclusion criteria required exposure within 72 hours prior to enrollment or within 24 hours for exposures involving high-risk areas such as the face, neck, hand, or fingers.
Participants were randomized in a 3:1 ratio to two groups: (1) RmAb (Rabishield at 3.33 IU/kg) plus PVRV (Rabivax-S) and (2) ERIG (Equirab at 40 IU/kg) plus PVRV. Both passive agents were infiltrated into the wound and its vicinity on day 0 as per WHO 2018 guidelines. Each group was further randomized (1:1) to receive PVRV either intradermally (0.1 mL + 0.1 mL on days 0, 3, 7, and 28) or intramuscularly (1.0 mL on days 0, 3, 7, 14, and 28).
The primary endpoint was the incidence of treatment-related serious adverse events (SAEs) up to 365 days post immunization, assessed in all treated participants. Secondary assessments included rabies virus neutralizing antibody (RVNA) geometric mean concentrations (GMCs) in a subset of participants and occurrence of rabies during the follow-up period.
Randomization employed permuted blocks with random block sizes of eight, and was managed centrally by an interactive web response system. The study was open-label, with no masking of participants or site personnel.
Key Findings
A total of 4059 participants were enrolled and randomized; 3994 received treatment (2996 in the RmAb plus PVRV group, 998 in the ERIG plus PVRV group). Among these, 3622 (90.7%) completed the full one-year follow-up.
Safety:
– Eleven adverse events (AEs) in the RmAb group and seventeen in the ERIG group were considered causally related to treatment.
– Most AEs were mild and transient, consistent with expected post-vaccination responses.
– Seven SAEs occurred within the RmAb group, all determined to be unrelated to treatment.
– One serious AE in the ERIG group was causally related to treatment.
Immunogenicity:
– On day 14 post-exposure, RVNA geometric mean concentrations rose to 16.05 IU/mL (95% CI 13.25–19.44) in the RmAb group and 13.48 IU/mL (95% CI 9.51–19.11) in the ERIG group.
– The point estimate ratio of GMCs was 1.19 (95% CI 0.82–1.72), indicating comparable immunogenicity between groups.
Efficacy:
– No cases of rabies were reported in any participants during the one-year follow-up, demonstrating effective protection by both regimens.
Additional subgroup analysis confirmed that route of vaccine administration (intradermal versus intramuscular) did not significantly affect immunogenicity or safety outcomes.
Expert Commentary
This study provides robust evidence supporting the use of a RmAb plus purified Vero cell rabies vaccine as an effective PEP strategy in patients with category 3 exposures to suspected rabid animals. The comparable immunogenic response and safety profile vis-à-vis the established ERIG regimen highlight the potential for RmAb to substitute traditional immunoglobulins, overcoming supply and adverse reaction limitations.
Notably, the stringent adherence to WHO 2018 guidelines for infiltration and vaccination schedules strengthens the clinical relevance of these findings. The open-label design may be considered a limitation due to potential assessment bias in adverse event reporting, but the large sample size and long follow-up enhance reliability.
The absence of rabies development among treated patients confirms the protective efficacy of this regimen in a high-risk population. Additionally, sustained antibody levels imply durable immunity, an important consideration for rabies prophylaxis.
These findings align with increasing global interest in monoclonal antibody therapies for infectious disease management, offering scalable, standardized passive immunization options.
Conclusion
Rabies monoclonal antibody combined with purified Vero cell rabies vaccine represents a safe, well-tolerated, and effective post-exposure prophylaxis for WHO category 3 rabies exposures. This phase 4 clinical trial conducted in India demonstrated long-term immunogenicity, a favorable safety profile, and zero rabies incidence during 12 months follow-up, meeting critical needs for improved passive immunization strategies.
Implementation of RmAb-based prophylaxis can enhance availability and standardization of rabies PEP globally, particularly in endemic regions with limited access to equine immunoglobulin.
Future research should explore cost-effectiveness, accessibility, and integration into regional rabies control programs, alongside monitoring real-world effectiveness across diverse populations.
References
Kulkarni PS, Potey AV, Kapse D, Bhamare C, Gawande A, Munshi R, Pawar S, Gogtay NJ, Agarwal A, Tambe M, Thakre S, Samuel CJ, Khan SMS, S RH, Rana D, Singh N, Kamath V, Bhalla HL, Poonawalla CS, Mani RS, Gunale B; RAB-04 study group. Post-exposure prophylaxis regimen of rabies monoclonal antibody and vaccine in category 3 potential exposure patients: a phase 4, open-label, randomised, active-controlled trial. Lancet. 2025 Aug 9;406(10503):627-635. doi: 10.1016/S0140-6736(25)00735-4. PMID: 40783290.
World Health Organization. WHO Expert Consultation on Rabies. Third report. WHO Technical Report Series, No. 1012. Geneva: WHO; 2018.