The Enigma of Aging
Aging is a complex, multi-faceted process that affects various organ systems within the human body. Despite extensive research, the precise molecular mechanisms driving aging remain largely elusive. A fundamental question persists: Do different organ systems age in a synchronized manner, or do they follow distinct timelines?
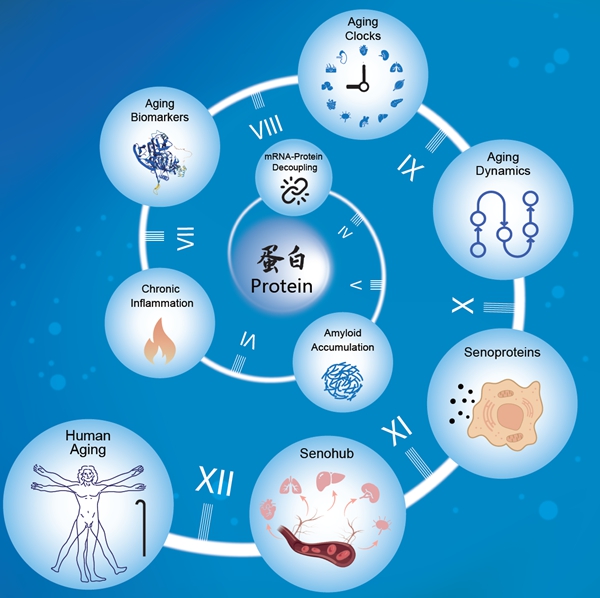
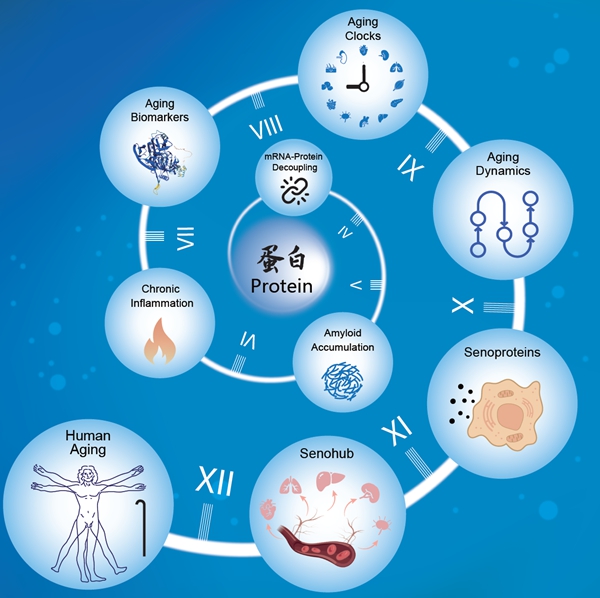
Recent scientific advancements have highlighted the importance of proteostasis—the balance of protein synthesis, folding, modification, transport, and degradation—in the aging process. Proteins, being essential to cellular structure and function, are integral to maintaining physiological homeostasis. Disruptions in proteostasis can lead to age-related dysfunction and diseases.
The Breakthrough Study
In a landmark study published in the journal Cell, a collaborative team of researchers, including Dr. Guanghui Liu from the Chinese Academy of Sciences and Dr. Weiqi Zhang from the National Center for Bioinformatics, utilized cutting-edge mass spectrometry and machine learning technologies to map the proteomic changes across human lifespans. Their research spanned 50 years of human life, analyzing seven major physiological systems and 13 key tissues to create a comprehensive “proteomic chronicle of aging.”
Key Findings: The Aging Blueprint
The study unveiled that disruptions in protein information flow represent a central feature of organ aging. This disruption is characterized by a decoupling of mRNA and protein abundance and pathological protein depositions, such as amyloid plaques. Notably, the vascular system emerged as a “pioneer” in the aging process, with significant deviations from homeostasis observed early in life.
Proteins such as GAS6, identified as “senoproteins,” play pivotal roles in triggering and amplifying systemic aging across organs. These proteins activate cross-organ signaling networks, functioning as a “senohub” to coordinate the aging process.
The Role of Proteostasis in Aging
The study further elucidated the mechanisms by which proteostasis is disturbed during aging. Three core manifestations were identified:
– Central Dogma Disruption: As people age, the correlation between mRNA and protein levels weakens, hindering the accurate translation of genetic information into functional proteins.
– Protein Quality Control Decline: The study noted a general downregulation of ribosomal proteins, molecular chaperones, and proteasomal subunits, suggesting a decline in the synthesis, folding, and degradation of proteins across various tissues.
– Pathological Protein Aggregation: Amyloid, immunoglobulins, and complement components accumulate abnormally in aging tissues, forming a pro-inflammatory network that underpins inflammaging.
Aging Clocks and Turning Points
Using advanced artificial intelligence algorithms, the researchers constructed a “proteomic aging clock” specific to 13 human tissues. Their analysis identified the age of 30 as a critical juncture, with the adrenal glands showing the earliest signs of aging, indicating endocrine imbalance as an early driver. The aorta also displayed early deviations, underscoring its role as an “aging sentinel.”
The period between 45 and 55 years of age was identified as a major turning point, where most organ systems experience a “molecular cascade storm,” with a surge in differentially expressed proteins marking a shift towards systemic aging.
Implications for Anti-Aging Strategies
The confirmation of the vascular system as a “central hub” in aging opens new avenues for therapeutic interventions. Targeting senoproteins and restoring proteostasis could offer novel strategies to delay or mitigate the effects of aging. Future research will likely focus on non-invasive biomarkers for aging detection and the development of organ-specific “aging clocks” for clinical applications.
Conclusion: Towards a New Paradigm
This pioneering research marks a significant step towards unraveling the complex mechanisms of aging. By integrating proteomic data, artificial intelligence, and functional validation, the study proposes a paradigm shift in aging research—from cellular mechanisms to inter-organ communication networks. Such insights hold promise for extending healthy lifespan and enhancing quality of life in aging populations.
References
Ding Y, Zuo Y, Zhang B, Fan Y, Xu G, Cheng Z, Ma S, Fang S, Tian A, Gao D, Xu X, Wang Q, Jing Y, Jiang M, Xiong M, Li J, Han Z, Sun S, Wang S, He F, Yang J, Qu J, Zhang W, Liu GH. Comprehensive human proteome profiles across a 50-year lifespan reveal aging trajectories and signatures. Cell. 2025 Jul 22:S0092-8674(25)00749-4. doi: 10.1016/j.cell.2025.06.047 .