Highlight
– Computational analysis of tubular features from whole slide images offers enhanced prognostic discrimination in FSGS and MCD compared to conventional assessments.
– Nine specific tubular features, including basement membrane thickness and epithelial flattening, correlate strongly with disease progression and proteinuria remission.
– Tubular morphology changes show a gradient with interstitial fibrosis severity, highlighting spatial relationships important for understanding pathophysiology.
– Integration of deep learning and pathomics enables quantitative, reproducible insights into tubular pathology beyond traditional visual scoring.
Study Background and Disease Burden
Focal segmental glomerulosclerosis (FSGS) and minimal change disease (MCD) are glomerular disorders presenting significant challenges due to their variable clinical courses and treatment responses. The prognosis is closely linked to tubular and interstitial injury, particularly interstitial fibrosis and tubular atrophy (IFTA). Conventional histopathologic evaluation relies on visual scoring, which may not capture subtle tubular alterations or the full structural spectrum predictive of outcomes. This limitation hinders accurate risk stratification and therapeutic decision-making. Enhanced techniques that provide quantitative and reproducible characterization of tubular changes and their spatial relationship with the interstitial microenvironment could improve prognostic accuracy and patient management.
Study Design
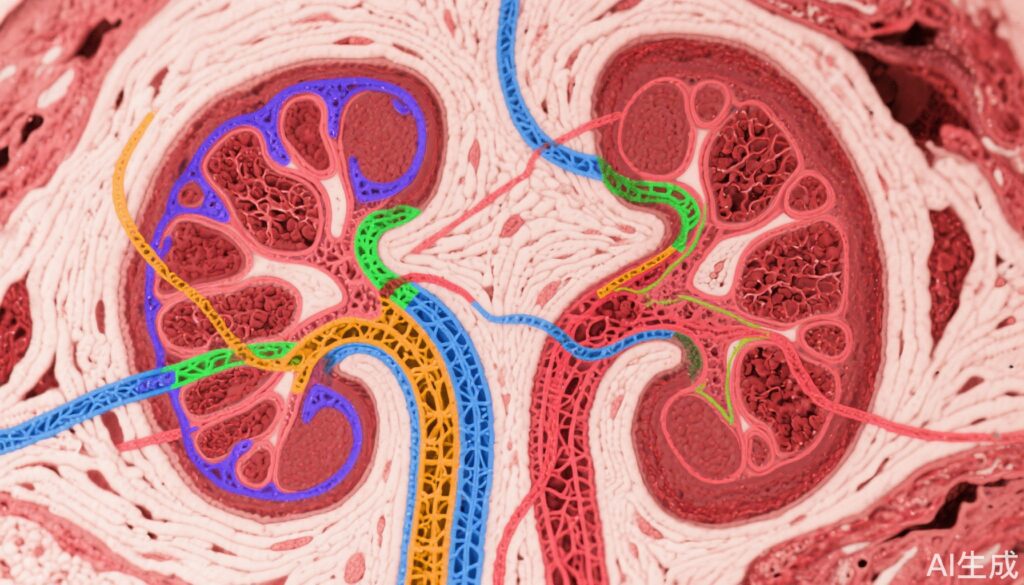
This investigation utilized deep learning and image analysis algorithms on Periodic acid Schiff (PAS)-stained whole slide images (WSIs) of kidney biopsies from 254 participants in the NEPTUNE and 266 in the CureGN prospective observational cohorts, including 135/153 with FSGS and 119/113 with MCD respectively. Cortical regions, tubular lumen (TL), tubular epithelium (TE), nuclei (TN), and tubular basement membrane (TBM) were segmented computationally. A total of 104 pathomic features capturing morphological and spatial characteristics were extracted and aggregated at the patient level.
In NEPTUNE, regions of mature IFTA, pre-IFTA, and non-IFTA were manually delineated to enable regional tubular feature quantification. Feature selection to identify those most associated with disease progression and proteinuria remission employed Minimum Redundancy Maximum Relevance (mRMR). Predictive performance was evaluated with ridge-penalized Cox regression models compared against traditional clinical, demographic, and visual pathology data and subsequently validated in the CureGN dataset.
Key Findings
Nine computationally derived tubular features demonstrated independent predictive value for progression of kidney disease and proteinuria remission. These features included measures of TBM thickness and area, TE flattening, and reduction of cell size, which showed progressive increases across non-IFTA, pre-IFTA, and mature IFTA regions.
Integration of tubular features into prognostic models yielded higher accuracy than conventional parameters alone in both NEPTUNE and CureGN cohorts, indicating strong external validity. Feature importance suggested that subtle, quantifiable changes in tubular morphology reflect underlying pathophysiology relevant for disease trajectory.
Moreover, spatial analyses highlighted that these tubular alterations are not uniformly distributed but correlate geographically with interstitial fibrosis severity, suggesting a microenvironmental interaction influencing disease progression.
Expert Commentary
This study represents a significant advance in renal pathology by using computational tools to overcome limitations of conventional visual scoring. The linkage of quantitative tubular structural features with meaningful clinical endpoints advances precision medicine approaches for glomerular diseases.
Notwithstanding these strengths, limitations include the retrospective analysis, the need for further validation across other kidney diseases and demographic groups, and the current requirement for high-resolution digital pathology infrastructure.
Mechanistically, the increase in TBM thickness and epithelial flattening aligns with known fibrotic remodeling processes, reinforcing biological plausibility. These insights may open avenues for targeted interventions that modulate interstitial-tubular interactions to alter disease course.
Conclusions
Computational quantification of tubular features from standard PAS-stained biopsies enhances prognostic stratification in FSGS and MCD beyond traditional clinical and histopathologic assessment. The ability to objectively measure spatially resolved tubular morphology offers new potential biomarkers for disease activity and prognosis.
Future prospective studies are warranted to validate these findings, explore application across broader nephropathies, and integrate computational pathology into routine clinical workflows for personalized management.
References
Fan F, Liu Q, Zee J, Ozeki T, Demeke D, Yang Y, Bitzer M, O’Connor CL, Farris AB, Wang B, Shah M, Jacobs J, Mariani L, Lafata KJ, Rubin J, Chen Y, Holzman LB, Hodgin JB, Madabhushi A, Barisoni L, Janowczyk A. Clinical relevance of computationally derived tubular features and their spatial relationships with the interstitial microenvironment in minimal change disease/focal segmental glomerulosclerosis. Kidney Int. 2025 Aug;108(2):293-309. doi: 10.1016/j.kint.2025.04.026. Epub 2025 May 21. PMID: 40409668; PMCID: PMC12302419.