Highlights
This single‑center randomized pilot (NCT04584346) examined feasibility, acceptability and short‑term physiologic and symptomatic effects of a medium‑chain triglyceride‑supplemented ketogenic diet (MCT‑KD) in 16 people with moderate Parkinson’s disease (PD).
– Primary feasibility achieved: 15/16 completed, mean acceptability 2.26/3, most participants adhered to target net carbohydrate 0.5 mM by inpatient day 4 in the KD group, rising to AM BHB ~1.25 mM on day 7 and 1.57 mM at week 3.
– No statistically significant improvement in the preplanned mobility endpoint (Timed Up & Go at day 7; KD 8.4 s vs SD 9.1 s, p = 0.23), but nonmotor symptoms (NMSS) improved overall (p = 0.04) and tended to improve more in KD.
– Short‑term metabolic benefits (weight loss, reduced triglycerides, increased HDL, lowered insulin and reduced respiratory quotient) were observed, with acceptable safety in this small cohort.
Study background and disease burden
Parkinson’s disease is a progressive neurodegenerative disorder characterised primarily by motor deficits (bradykinesia, rigidity, tremor, postural instability) and a wide range of nonmotor symptoms (fatigue, sleep disturbance, autonomic dysfunction, cognitive impairment). Disease‑modifying therapies remain lacking. Mitochondrial dysfunction, oxidative stress and impaired cellular energetics are implicated in PD pathogenesis, offering a rationale for metabolic interventions that supply alternative fuels and alter cellular signaling. Ketogenic diets, and exogenous/ dietary MCT formulations that rapidly raise circulating ketones, have been proposed to improve bioenergetic flux, reduce oxidative stress and modulate inflammation — mechanisms potentially relevant to PD symptom burden and progression.
Study design
This was a pilot feasibility randomized controlled trial conducted at the NIH Clinical Center. Key features:
– Population: 16 adults >50 years with clinically probable PD (Hoehn & Yahr 2–3, MoCA ≥21), recruited 2020–2021. One subject withdrew after the inpatient phase; 15 completed the study.
– Intervention: 1‑week double‑blind, inpatient randomized diet (MCT‑supplemented ketogenic diet [KD] vs isocaloric standard diet [SD]) followed by a 2‑week open‑label outpatient MCT‑KD for all participants.
– Diet specifics: KD provided ~80% energy from fat (≈25% of fat as Liquigen® MCT oil), 10–15% protein, and 5–10% net carbohydrate (target 20–50 g). SD provided ~35% fat and 50–55% carbohydrate. Meals were weighed and double‑blinded; outpatient KD used a study cookbook and provided MCT oil.
– Primary endpoint: feasibility composite (recruitment target, retention >80%, outpatient mean net carbohydrate <10% energy, exit acceptability Likert ≥2/3).
– Secondary (prespecified) endpoint: difference in Timed Up & Go (TUG) at inpatient day 7.
– Exploratory outcomes: blood ketones (BHB, acetoacetate), levodopa metabolites, UPDRS, NMSS, rsEEG connectivity, metabolic labs (lipids, insulin), continuous glucose monitoring, cognitive and motor tests.
Key findings
Feasibility and adherence
– Retention: 15/16 (94%) completed the study; primary composite feasibility criteria were met. Exit acceptability averaged 2.26/3, with 12/15 reporting they were somewhat‑to‑very likely to continue a similar KD.
– Dietary adherence: Mean reported net carbohydrate during the 2‑week at‑home KD was 9.7% (±3.1%) of energy; 9/15 participants maintained mean net carbs <10%.
Ketosis and metabolic effects
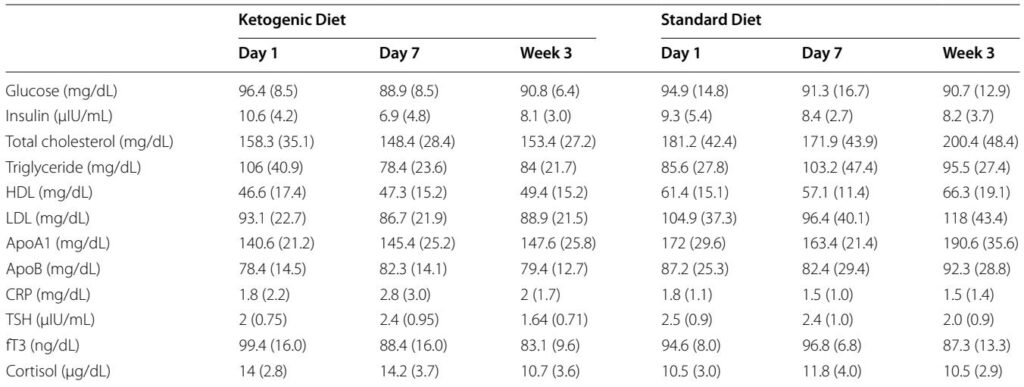
– Ketone kinetics: AM BHB in the KD group rose from baseline (~0.03 mM) to 0.59 ± 0.25 mM on day 4, 1.25 ± 0.70 mM on day 7, and 1.57 ± 1.05 mM at week 3, confirming rapid induction and maintenance of nutritional ketosis with MCT supplementation. SD group BHB remained near baseline.
– Glycemic and insulin response: Continuous glucose monitoring showed steady glucose profiles (60–140 mg/dL) with fewer post‑prandial spikes in KD versus SD. Insulin decreased in the KD group (day 1 mean 10.6 μIU/mL → day 7 6.9 μIU/mL → week 3 8.1 μIU/mL).
– Lipids and body composition: Both groups lost weight (mean loss pooled ≈2.6 ± 1.3 kg; p<0.0001), with reduced waist circumference and decreased triglycerides and modest HDL increases; changes were numerically greater in KD. Respiratory quotient fell from 0.80 to 0.73 (p≈0.000003), consistent with greater fat oxidation.
Motor and nonmotor outcomes
– Prespecified mobility outcome (TUG): Mean TUG improved modestly in both groups from baseline to day 7 (KD: 8.7 → 8.4 s; SD: 9.0 → 9.1 s). Adjusted analysis found no significant difference between KD and SD on day 7 (p = 0.23). The interim futility criterion for the planned larger trial was met and the study was stopped after 16 participants.
– UPDRS: No statistically significant change in UPDRS‑3 motor scores between groups during this short intervention.
– Nonmotor symptoms: NMSS scores declined for the entire cohort from 43.8 ± 17.6 to 31.5 ± 13.2 (p = 0.04). KD participants showed a numerically larger fall (46.1 → 24.4) than SD (43.8 → 33.0), with improvements concentrated in GI, urinary and miscellaneous domains; sleep/fatigue worsened in some KD subjects.
– Cognitive measures and fine motor tests: Both groups showed practice‑related improvements on certain tests (3‑back, Stroop, keyboard tasks). The KD group had a non‑controlled improvement on the nondominant 9‑hole pegboard.
Levodopa metabolism, EEG and other biomarkers
– Levodopa metabolites: Plasma dopamine and DOPAC showed nonsignificant trends to increase in KD versus SD over days 1–3 (dopamine p = 0.23; DOPAC p = 0.06), warranting further exploration of diet–drug–microbiome interactions.
– Resting‑state EEG: KD was associated with decreased delta and increased relative beta power and increased graph‑theory connectivity metrics (weighted kappa, clustering), suggesting acute effects on network physiology, though clinical significance is unclear.
Safety and tolerability
– Adverse events: Most common were fatigue (n=5), headache/light‑headedness (n=2), GI complaints (constipation, indigestion), and one case of diarrhea. One participant developed deep vein thrombosis/pulmonary embolus during the home KD phase; independent review judged this unlikely related to the diet. No serious metabolic derangements (severe hyperlipidemia, ketoacidosis) were reported.
Expert commentary and mechanistic considerations
Mechanistically, ketogenic interventions supply BHB and acetoacetate as alternative cerebral fuels, which may (1) improve mitochondrial ATP generation, (2) reduce reactive oxygen species, (3) modulate neurotransmitter balance (GABA/glutamate), and (4) exert anti‑inflammatory signaling (BHB inhibits the NLRP3 inflammasome). Preclinical models have shown benefits of BHB on mitochondrial respiration in PD models (Tieu et al., 2003), and human studies in other neurodegenerative conditions have reported short‑term cognitive benefit with ketone elevation. The observed metabolic benefits (improved insulin, triglycerides, HDL) align with established KD physiology and may be especially relevant in PD patients with metabolic syndrome.
The trend toward increased peripheral dopamine/DOPAC in the KD group raises an intriguing but unresolved possibility: diet‑driven shifts in gut microbiota or peripheral decarboxylation may alter levodopa availability. Conversely, EEG network changes (increased beta and connectivity) could reflect altered cortical excitability or neurotransmitter balance and might be a candidate noninvasive biomarker for future studies.
Limitations and generalizability
This pilot trial was small (N=16) and short (3 weeks), and was not powered to detect modest clinical motor effects. All assessments were performed in the dopaminergic medication “ON” state and several participants had DBS, which may have limited the ability to detect incremental motor benefits. The inpatient alimentary environment and the study‑specific KD cookbook differ from real‑world practice, and dietary recall (used for outpatient adherence assessment) may underestimate intake. Baseline group imbalances (e.g., disease duration, LEDD, DBS frequency) and the decision to convert all subjects to open‑label KD after day 7 limit between‑group comparisons at week 3. Finally, EEG and metabolite signals were exploratory and require replication.
Conclusions and implications for clinical practice
A medium‑chain triglyceride–supplemented ketogenic diet is feasible and acceptable to motivated people with moderate PD, produces rapid nutritional ketosis and short‑term metabolic benefits, and may improve certain nonmotor domains. In this small, short trial there was no significant improvement in a prespecified mobility outcome (TUG) at day 7. The safety profile was acceptable in this selected cohort but included expected keto‑induction symptoms and one unrelated thromboembolic event.
For clinicians: MCT‑KD can be considered investigationally for motivated PD patients — particularly when targeting nonmotor symptoms or metabolic comorbidities — but evidence for motor improvement or disease‑modifying effect is not established. If attempted, careful baseline screening (renal, hepatic, lipid profile), dietetic support, and monitoring (ketones, lipids, glucose/insulin, hydration, and medication timing) are advisable.
Research priorities: Larger, longer randomized trials should (1) test clinically meaningful motor and cognitive endpoints over months, (2) stratify by metabolic phenotype (insulin resistance), (3) evaluate interactions with levodopa pharmacokinetics and the gut microbiome, and (4) validate EEG or other biomarkers as objective readouts of brain effects.
Selected references
1. Tieu K, Perier C, Caspersen C, et al. D‑beta‑Hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Invest. 2003;112(6):892–901.
2. Vanitallie TB, Nonas C, Di Rocco A, et al. Treatment of Parkinson disease with diet‑induced hyperketonemia: a feasibility study. Neurology. 2005;64:728–730.
3. Phillips MCL, Murtagh DKJ, Gilbertson LJ, et al. Low‑fat versus ketogenic diet in Parkinson’s disease: A pilot randomized controlled trial. Mov Disord. 2018;33:1306–1314.
4. Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite beta‑hydroxybutyrate blocks NLRP3 inflammasome‑mediated inflammatory disease. Nat Med. 2015;21:263–269.
5. Maini Rekdal V, Bess EN, Bisanz JE, Turnbaugh PJ, Balskus EP. Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science. 2019;364(6445):eaau6323.
(For full trial protocol and additional data see ClinicalTrials.gov NCT04584346.)