Highlight
- Serum plasmalogen levels and gut microbiota profiles strongly associate with seizure reduction in children with drug-resistant epilepsy on the ketogenic diet.
- Positive correlation of anti-seizure effect with Faecalibacterium prausnitzii, Alistipes spp., and Christensenella minuta; negative correlation with Escherichia coli strains.
- Potential for targeted microbiome or metabolite therapies to enhance seizure control.
Study Background and Disease Burden
Drug-resistant epilepsy affects up to 30% of children with epilepsy, representing a significant clinical challenge due to persistent seizures and associated neurocognitive, psychosocial, and physical morbidity. The ketogenic diet (KD)—a high-fat, adequate-protein, and low-carbohydrate regimen—has long been employed when pharmacologic options fail, yet its mechanisms remain incompletely understood. Recent preclinical data highlight the critical role of gut microbiota and circulating metabolites, suggesting a complex interplay between diet, microbial communities, and neurobiology. Bridging these mechanistic insights to clinical practice could unlock new therapeutic avenues for refractory epilepsy.
Study Design
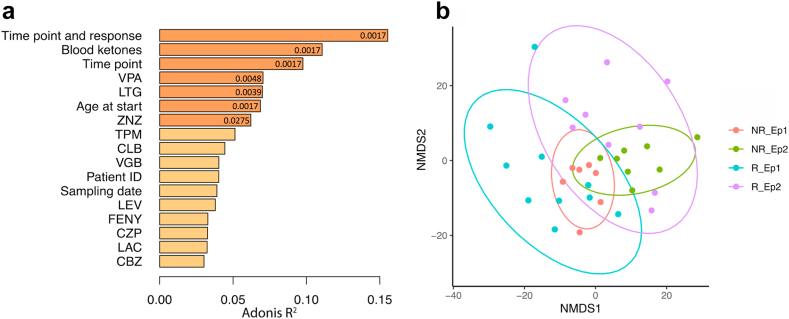
This prospective observational study enrolled 14 children with drug-resistant epilepsy. Serum metabolome and fecal microbiome samples were collected before and after three months of KD therapy. The primary endpoint was seizure reduction, assessed in relation to metabolomic and microbiome shifts. Correlations between specific serum metabolites, gut microbial taxa, and clinical outcomes were analyzed to elucidate mechanistic associations.
Key Findings
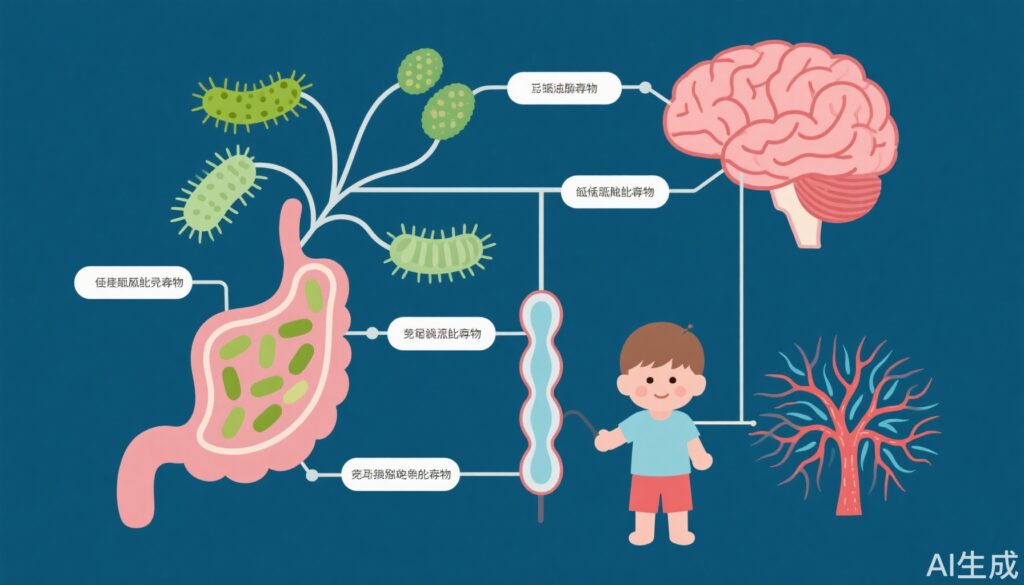
The study revealed strong associations between changes in serum metabolites and gut microbiota composition with seizure reduction during KD therapy:
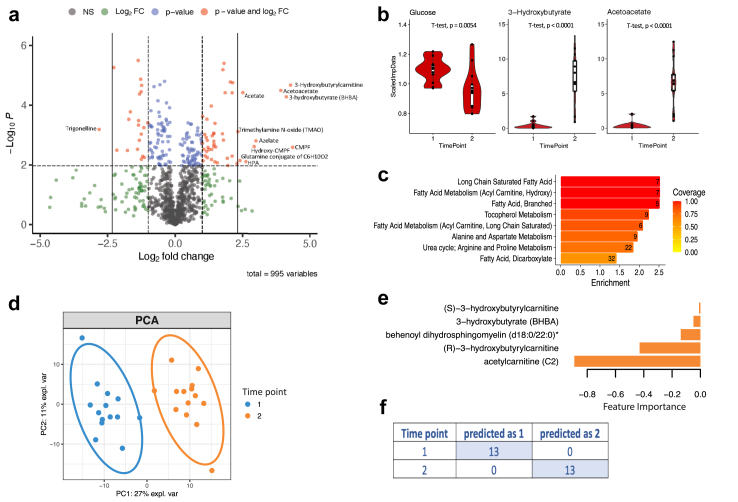
- Plasmalogens: These ether phospholipids were most strongly linked to seizure reduction. Plasmalogens are known to influence neuroinflammation, membrane integrity, and synaptic function—key processes in seizure pathophysiology.
- Gut Microbiota: Several beneficial bacterial species, including Faecalibacterium prausnitzii, Alistipes communis, Alistipes shahii, and Christensenella minuta, showed significant positive correlations with plasmalogen levels and seizure reduction. In contrast, five strains of Escherichia coli were negatively correlated with anti-seizure effects, potentially reflecting a pro-inflammatory or dysbiotic state.
- Infant-type Bifidobacteria: These correlated negatively with metabolites associated with seizure reduction, suggesting age- or diet-dependent microbial dynamics may modulate therapeutic response.
- Metabolomic-Microbiome Correlation: The integrative analysis underscores the complex bidirectional relationship between the gut microbiome and host metabolome in the context of KD, implicating microbial modulation as a key driver of the anti-seizure effect.
Serum metabolomic profiles of patients with epilepsy are influenced by treatment and response.
The ketogenic diet induces metabolomic changes in the serum of children with severe epilepsy.
The study did not report significant adverse effects related to KD or microbiota alterations, but the small sample size precludes definitive safety conclusions.
Expert Commentary
Current clinical guidelines endorse the KD for drug-resistant epilepsy but do not specify microbiota or metabolite monitoring. This study advances the mechanistic understanding by linking specific bacterial taxa and metabolites—particularly plasmalogens—to seizure control. Plasmalogens, previously implicated in neurodegenerative and psychiatric disorders, may represent a promising biomarker or therapeutic target. The observed negative associations with E. coli strains and Bifidobacteria warrant further exploration, as these findings challenge conventional notions about ‘beneficial’ microbes across pediatric age groups and clinical contexts.
Limitations include the small cohort, short follow-up, and lack of mechanistic intervention (e.g., probiotic supplementation), limiting generalizability. Nonetheless, the integration of metabolomic and microbiome data in a clinical setting is an important step toward personalized anti-seizure strategies.
Conclusion
This study demonstrates that specific changes in serum metabolites and gut microbiota composition are associated with seizure reduction in children undergoing KD therapy for drug-resistant epilepsy. Plasmalogens and select gut microbes may mediate or predict anti-seizure efficacy, suggesting that metabolic and microbial profiling could inform future individualized treatment strategies. Further trials are warranted to validate these findings, elucidate causality, and explore targeted microbiota or metabolite-based interventions as adjuncts to dietary therapy.
References
- Dahlin M, Wheelock CE, Prast-Nielsen S. Association between seizure reduction during ketogenic diet treatment of epilepsy and changes in circulatory metabolites and gut microbiota composition. EBioMedicine. 2024 Nov;109:105400. doi: 10.1016/j.ebiom.2024.105400 IF: 10.8 Q1 . PDF (1.2 MB)
- Neal EG, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7(6):500-506.
- Olson CA, et al. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell. 2018;173(7):1728–1741.e13.