Highlights
- Combination of azacitidine, venetoclax, and revumenib achieved an 88.4% overall response rate in older adults with newly diagnosed NPM1-mutated or KMT2A-rearranged AML.
- No refractory disease observed after 1–2 cycles; most remissions achieved within the first cycle.
- Safety profile was manageable, with differentiation syndrome and QTc prolongation not requiring treatment discontinuation.
- All evaluated responders achieved undetectable measurable residual disease by centralized flow cytometry.
Study Background and Disease Burden
Acute myeloid leukemia (AML) is a highly aggressive hematologic malignancy, disproportionately affecting older adults. Despite the introduction of less intensive regimens, such as the combination of azacitidine and venetoclax, long-term outcomes for patients aged 60 and above—particularly those with adverse molecular features like NPM1 mutations (NPM1m) or KMT2A rearrangements (KMT2Ar)—remain unsatisfactory. These genetic subtypes confer unique biological vulnerabilities, yet also present therapeutic challenges, as relapse rates are high and durable remissions are elusive. The emergence of targeted agents such as revumenib, a potent oral menin inhibitor, offers a new avenue to exploit disease biology and potentially improve outcomes in these high-risk populations.
Study Design
This investigation was a phase I, open-label, dose-escalation and expansion trial (ClinicalTrials.gov: NCT03013998) that evaluated the safety and efficacy of combining azacitidine, venetoclax, and revumenib in newly diagnosed AML patients aged 60 years and older with either NPM1m or KMT2Ar. Revumenib was administered at two dose levels (113 mg or 163 mg orally every 12 hours) in conjunction with strong CYP3A4-inhibiting azole antifungals, alongside standard doses of azacitidine and venetoclax. The primary objectives were to determine the recommended phase II dose and assess safety; secondary endpoints included response rates, time to response, duration of remission, and measurable residual disease (MRD) status.
Key Findings
A total of 43 patients were enrolled and treated across both dose levels. Notably, no maximum tolerated dose (MTD) of revumenib was defined, supporting the feasibility of the regimen. The safety profile was generally acceptable:
- Differentiation syndrome occurred in 8 patients (19%), a well-recognized risk with menin inhibition, but was manageable and did not necessitate permanent discontinuation.
- QTc prolongation (Fridericia correction) was observed in 19 patients (44%), but again, none required permanent cessation of revumenib.
Efficacy results were particularly notable in this high-risk, older cohort:
- Overall response rate (ORR) was 88.4% (95% CI, 74.9–96.1%). Stratified by genotype, ORR was 85.3% for NPM1m and 100% for KMT2Ar patients.
- Composite complete remission (CR + CR with partial/incomplete hematologic recovery) was 81.4% (95% CI, 66.6–91.6%), with CR alone achieved in 67.4% (95% CI, 51.5–80.9%).
- No patients exhibited refractory disease following 1–2 cycles of therapy.
- The median time to first response was 28 days, with 84% of responders entering remission within the first cycle—suggesting rapid disease control.
- All 37 evaluable patients achieved MRD negativity as assessed by high-sensitivity centralized flow cytometry—a promising surrogate for durable remission.
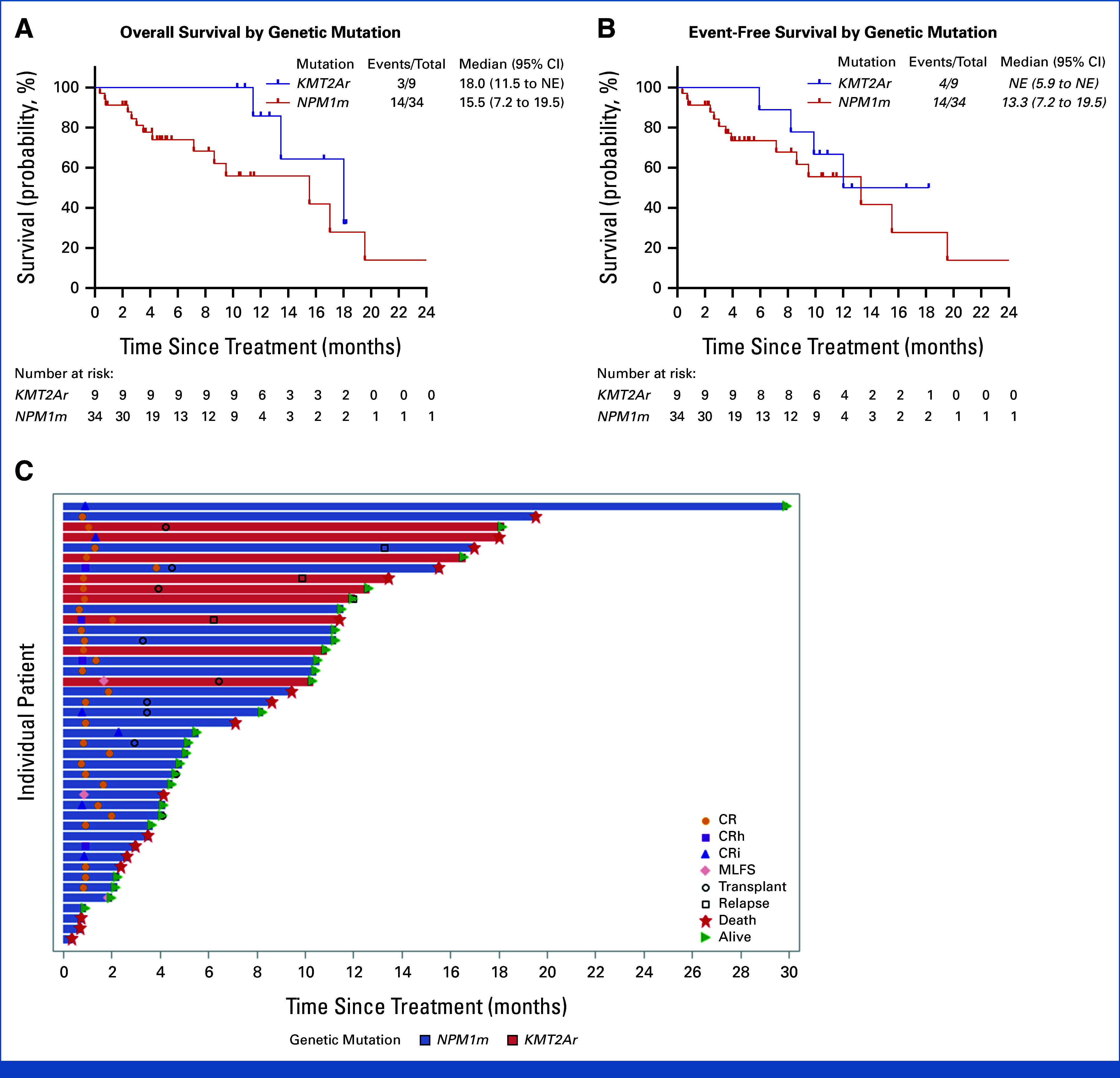
Overall and event-free survival. (A) Median overall survival in KMT2Ar was 18.0 months (95% CI, 11.5 to not reached) and that in NPM1m was 15.5 months (95% CI, 7.2 to 19.5). (B) Median event-free survival in KMT2Ar was not reached and that in NPM1m was 13.3 months (95% CI, 7.2 to 19.5). (C) Swimmer plot describing response, relapse, transplant, and death.
There were no new or unexpected safety signals. The combination therapy did not result in cumulative toxicity, and dose interruptions or modifications were readily managed. Importantly, the results were consistent across both genetic subtypes, highlighting the potential of menin inhibition as a broadly effective strategy in molecularly defined AML.
Expert Commentary
The integration of revumenib, an oral menin inhibitor, into the standard azacitidine-venetoclax backbone marks a notable advance for NPM1m and KMT2Ar AML, two subgroups with historically poor prognosis on conventional regimens. The rapid achievement of deep remissions—including universal MRD negativity among those assessed—suggests this triplet regimen may alter the disease trajectory for many patients. While phase I data are inherently preliminary and limited by sample size, the absence of refractory disease after initial cycles and the manageable toxicity profile are encouraging.
Limitations include the small cohort and short follow-up, precluding definitive conclusions regarding overall survival or late toxicities. Questions remain regarding the durability of MRD negativity, the optimal sequencing of therapy for patients eligible for transplant, and how best to manage the observed cardiac effects in broader practice. Nonetheless, these results have informed ongoing phase II/III studies and may ultimately reshape the frontline management of molecularly characterized AML.
Mechanistically, menin inhibition disrupts leukemogenic transcriptional programs driven by both NPM1 mutations and KMT2A fusions, offering a rational, biology-driven combination with established hypomethylating and BCL-2 inhibition. This approach exemplifies the promise of precision medicine in AML.
Conclusion
The combination of azacitidine, venetoclax, and revumenib is safe, highly active, and capable of inducing rapid, deep remissions in older adults with newly diagnosed NPM1-mutated or KMT2A-rearranged AML. While longer-term follow-up and larger randomized studies are needed, these findings represent an important step toward improving outcomes in high-risk AML. Clinicians should remain attuned to emerging data and consider clinical trial enrollment for eligible patients, as the therapeutic landscape for AML continues to evolve.
References
Zeidner JF, Lin TL, Welkie RL, Curran E, Koenig K, Stock W, Madanat YF, Swords R, Baer MR, Blum W, Stein EM, Olin RL, Schiller G, Nichols A, Odenike O, Traer E, Lachowiez C, Duong VH, Hochman MJ, Cai SF, Smith C, Stefanos M, Martycz M, Huang Y, Rosenberg L, Marcus S, Chen TL, Yocum AO, Druker BJ, Levine RL, Borate U, Byrd JC, Mims AS. Azacitidine, Venetoclax, and Revumenib for Newly Diagnosed NPM1-Mutated or KMT2A-Rearranged AML. J Clin Oncol. 2025 Aug 10;43(23):2606-2615. doi: 10.1200/JCO-25-00914 IF: 41.9 Q1 .