Highlights
- The ARON cluster-randomised trial (2021–2023) showed a 27% relative reduction in antibiotic prescribing at index consultation among acutely ill children using a clinical decision tool integrating a decision tree, point-of-care C-reactive protein (CRP) testing, and safety-netting advice.
- The intervention did not prolong recovery time, increase additional testing, follow-up visits, or antibiotic prescribing after index consultation, demonstrating non-inferiority for key safety and healthcare utilization outcomes.
- Qualitative data highlighted the utility of CRP POCT in reducing diagnostic uncertainty and managing parental expectations, emphasizing clinician and caregiver acceptability.
- Prior evidence supports CRP POCT’s role in reassuring physicians, especially with normal CRP levels discouraging antibiotic use, though benefits are enhanced with clinical guidance and safety-netting.
Background
Antimicrobial resistance (AMR) poses a critical global health threat exacerbated by inappropriate antibiotic prescribing, particularly in pediatric ambulatory care where many children presenting with acute infections receive antibiotics unnecessarily. Children frequently present to primary care with self-limiting conditions, often viral, for which antibiotics provide no clinical benefit but risk resistance development and adverse effects. Strategies to optimize antibiotic use include clinical decision tools combining risk stratification algorithms, biomarkers like CRP tested at point-of-care, and clear safety-netting advice to guide watchful waiting. Belgium’s ARON study rigorously evaluated such an intervention’s effectiveness and safety in primary care settings.
Key Content
1. The ARON Trial: Design and Principal Findings
The ARON (Antibiotic Prescribing Rate after Optimal Near-patient Testing) trial was a pragmatic, unblinded, cluster-randomised controlled trial conducted in Belgian primary care between February 2021 and December 2023 (ClinicalTrials.gov NCT04470518). Eligible general practitioner and community pediatrician practices not already using CRP POCT were randomized 1:1 to implement a clinical decision tool or to continue usual care.
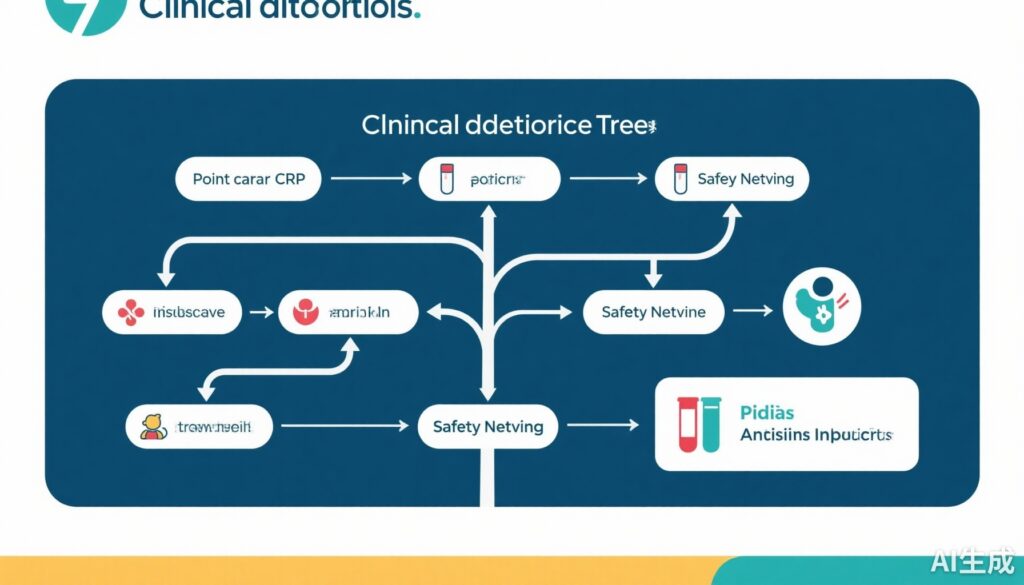
The intervention comprised three components: 1) a validated clinical decision tree to stratify infection severity; 2) guided use of point-of-care CRP testing to support clinical judgment; and 3) safety-netting advice to caregivers addressing illness progression and when to seek further care. Children aged 6 months to 12 years presenting with acute illness were consecutively recruited and followed for 30 days.
Primary outcomes were antibiotic prescribing at index consultation (superiority tested) and clinical recovery time, additional testing, follow-up visits, and antibiotic prescribing after index consultation (non-inferiority tested with predefined margins). Analysis accounted for clustering and adjusted for age.
Among 6750 participants analyzed (2988 intervention, 3762 control), antibiotic prescribing at index consultation was significantly reduced (16% vs. 22%; adjusted odds ratio 0.72, 95% CI 0.55-0.94; p=0.017). Recovery time (-0.1 day, 95% CI -0.5 to 0.3) and secondary outcomes were non-inferior. Serious adverse events occurred less frequently in the intervention arm and none were attributed to the intervention. No deaths occurred.
2. Complementary Qualitative Insights on CRP POCT and Safety-Netting
A nested process evaluation involving interviews with general practitioners and parents in the intervention arm (JAC Antimicrobial Resistance, 2024) elucidated behavioral mechanisms. Themes identified included CRP POCT supporting diagnostic confidence, modulating antibiotic prescriptions by alleviating parental pressure, and safety-netting enhancing parental self-management and reassurance.
Interestingly, intermediate CRP values were sometimes interpreted as indicators of possible serious infection, diverging from protocol intentions as a rule-out tool. The parental information booklet was valued as both an educational and emotional support tool.
3. CRP POCT in Pediatric Primary Care: Context and Prior Evidence
Earlier randomized controlled studies have demonstrated that use of CRP POCT alone without clinical algorithms does not reliably reduce antibiotic prescribing in children (Arch Dis Child 2016; Scand J Prim Health Care 2018). However, when used with clear clinical guidance and safety-netting strategies, CRP testing can influence physician decision-making by reducing diagnostic uncertainty and encouraging antibiotic withholding, especially when CRP levels are low.
A large RCT in Latvia demonstrated regional variability in antibiotic prescribing and CRP testing use; educational interventions combined with CRP POCT reduced prescribing significantly only in rural settings. Multiplex PCR testing for respiratory pathogens, while informative, did not significantly reduce antibiotic use in pediatric emergency care in Finland, underscoring the need for targeted biomarker-guided approaches optimized for primary care contexts.
4. Mechanistic and Translational Implications
CRP is an acute-phase reactant produced in response to pro-inflammatory cytokines, reflecting bacterial infection severity. Sensitive, rapid POCT facilitates real-time risk stratification, enabling early identification of children unlikely to benefit from antibiotics. Integration into decision trees contextualizes CRP values within clinical assessment, preventing overreliance on isolated biomarker thresholds and reducing false positives.
Safety-netting is an essential adjunct translating diagnostic certainty into safe care pathways, empowering parents to monitor illness progression and return if necessary, mitigating risks of delayed serious illness recognition.
Expert Commentary
The ARON trial provides robust evidence supporting multidimensional clinical decision tools that combine physician judgment, biomarker use, and caregiver communication to safely reduce unnecessary antibiotic exposure in children presenting to primary care. Compared with prior studies assessing isolated biomarker tests, ARON’s pragmatic design and large sample size enhance external validity.
Limitations include unblinded design and potential practice-level variations in intervention fidelity. The finding that intermediate CRP levels were sometimes misinterpreted suggests ongoing needs for clinician education and algorithm refinement.
Current pediatric stewardship guidelines increasingly recognize POCT combined with clinical algorithms as effective means to optimize antibiotic use. Yet real-world implementation must address barriers such as clinician trust, workflow integration, and parental education.
Future research should address long-term impact on antimicrobial resistance patterns, cost-effectiveness analyses, and adaptation to different healthcare settings. Additionally, exploration of adjunctive molecular diagnostics might tailor stewardship more precisely, although their added value over CRP-based tools requires confirmation.
Conclusion
The ARON trial convincingly demonstrates that a clinical decision tool integrating a validated decision tree, point-of-care CRP testing, and safety-netting advice reduces antibiotic prescribing rates in acutely ill children without compromising recovery or safety, supporting broader dissemination in primary care.
This composite approach mitigates diagnostic uncertainty and addresses parental concerns, key drivers of antibiotic overprescribing. Coupled with qualitative evidence underscoring acceptability and behavioral mechanisms, the data provide a strong foundation for clinical guideline incorporation and health policy initiatives to combat pediatric antibiotic overuse in ambulatory care.
Ongoing education to optimize test interpretation and implementation fidelity will be critical for sustained impact.
References
- Verbakel JY, Burvenich R, D’hulster E, et al. A clinical decision tool including a decision tree, point-of-care testing of CRP, and safety-netting advice to guide antibiotic prescribing in acutely ill children in primary care in Belgium (ARON): a pragmatic, cluster-randomised, controlled trial. Lancet. 2025 Sep 25:S0140-6736(25)01239-5. doi:10.1016/S0140-6736(25)01239-5. PMID:41016406.
- Burkens O, Verschuere S, Verbakel JY. Behavioural impact of antibiotic stewardship in children in primary care: interviews with GPs and parents. JAC Antimicrob Resist. 2024;6(6):dlae207. doi:10.1093/jacamr/dlae207. PMID:39691791.
- Straupe I, et al. Impact of educational training and C-reactive protein point-of-care testing on antibiotic prescribing in rural and urban family physician practices in Latvia: randomized controlled intervention study. BMC Pediatr. 2022;22(1):556. doi:10.1186/s12887-022-03608-4. PMID:36127630.
- Toivonen L, et al. Effect of Point-of-Care Testing for Respiratory Pathogens on Antibiotic Use in Children: A Randomized Clinical Trial. JAMA Netw Open. 2022;5(6):e2216162. doi:10.1001/jamanetworkopen.2022.16162. PMID:35679047.
- Verbakel JY, et al. Antibiotic prescribing rate after optimal near-patient C-reactive protein testing in acutely ill children presenting to ambulatory care (ARON project): protocol for a cluster-randomized pragmatic trial. BMJ Open. 2022;12(1):e058912. doi:10.1136/bmjopen-2021-058912. PMID:34980633.
- Bruins Slot KM, et al. Point-of-care CRP matters: normal CRP levels reduce immediate antibiotic prescribing for acutely ill children in primary care: a cluster randomized controlled trial. Scand J Prim Health Care. 2018;36(4):423-436. doi:10.1080/02813432.2018.1529900. PMID:30354904.
- Van den Bruel A, et al. C-reactive protein point-of-care testing in acutely ill children: a mixed methods study in primary care. Arch Dis Child. 2016;101(4):382-385. doi:10.1136/archdischild-2015-309228. PMID:26757989.
- Huttner B, Goossens H, Verheij T, Harbarth S. Characteristics and Outcomes of Public Campaigns Aimed at Improving the Use of Antibiotics in Outpatient Care. Lancet Infect Dis. 2010;10(1):17-31. doi:10.1016/s1473-3099(09)70305-2. PMID:20004463.