Highlight
1. Renal denervation (RDN) achieved significantly greater reductions in both 24-hour ambulatory and office systolic blood pressure at 24 months compared with sham control.
2. The control group increased antihypertensive medication use more than the RDN group over 24 months.
3. No serious adverse renal events, including renal artery stenosis, were reported through 24 months, confirming long-term safety.
4. Sensitivity analyses accounting for crossover patients validated the robustness of the blood pressure benefits with RDN.
Study Background and Disease Burden
Hypertension remains a leading modifiable risk factor for cardiovascular morbidity and mortality worldwide. Although pharmacologic therapy effectively lowers blood pressure in most patients, many experience insufficient control or intolerance to medications, resulting in persistent elevated cardiovascular risk. Renal denervation (RDN) — a catheter-based procedure targeting renal sympathetic nerves — has emerged as a promising adjunct therapy for treatment-resistant or uncontrolled hypertension. Earlier trials showed mixed results, partly due to patient selection and medication confounders.
The SPYRAL HTN-ON MED trial explored RDN using the Symplicity Spyral multi-electrode system in patients on 1 to 3 antihypertensive drugs. Initial six-month results showed RDN reduced office blood pressure but did not significantly lower 24-hour ambulatory systolic pressure compared to sham. Given the chronic nature of hypertension and the possibility of delayed effects, the present prespecified analysis investigates the long-term efficacy, safety, and changes in medication use through 24 months.
Study Design
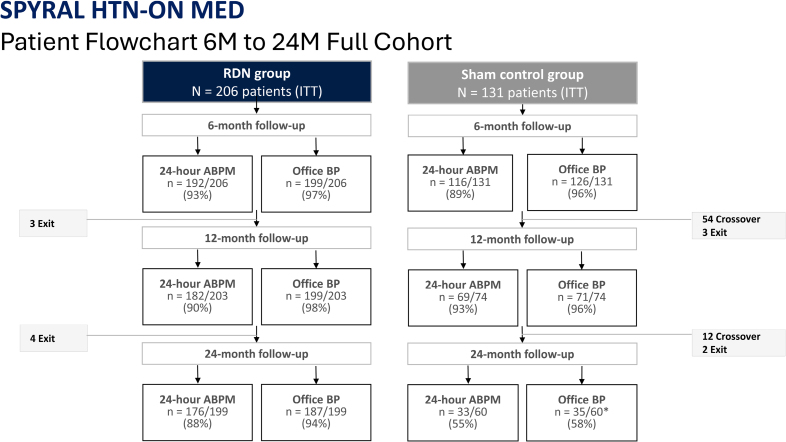
This prospective, randomized, sham-controlled, blinded trial enrolled 337 hypertensive patients from 56 global centers. Eligibility criteria included office systolic blood pressure between 150 and 180 mm Hg, diastolic blood pressure ≥90 mm Hg, and 24-hour ambulatory systolic blood pressure between 140 and 170 mm Hg, while patients were receiving stable antihypertensive therapy (1–3 agents) for at least 6 months.
Participants were randomized to RDN or a sham procedure. Both groups continued their baseline medication regimen for 6 months under blinded conditions. Subsequently, patients and investigators were unblinded; changes to antihypertensive therapy were allowed, and control group patients could cross over to receive RDN. Crossover patients’ last observations were carried forward in the control analysis. The study’s primary endpoints were changes in 24-hour ambulatory and office systolic blood pressure at 24 months, alongside evaluation of medication use and safety outcomes.
Key Findings
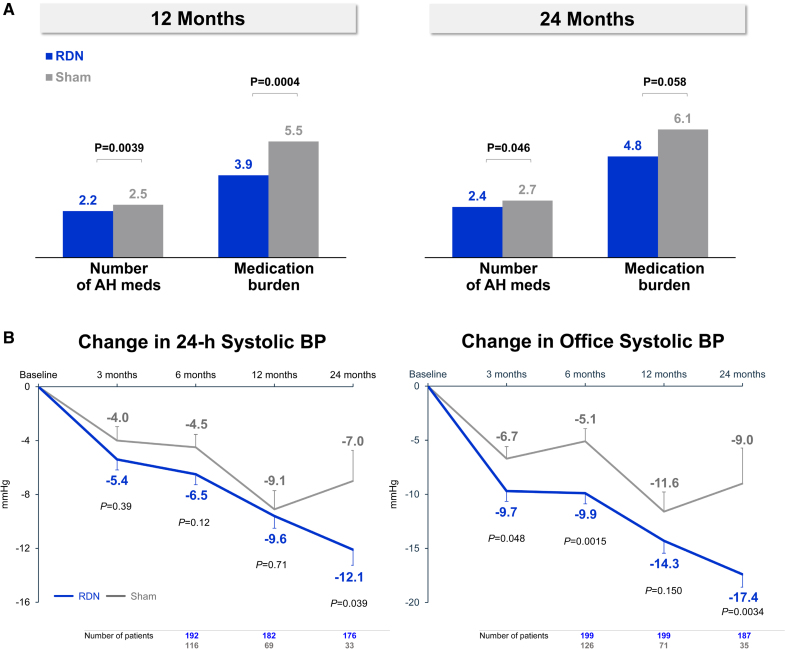
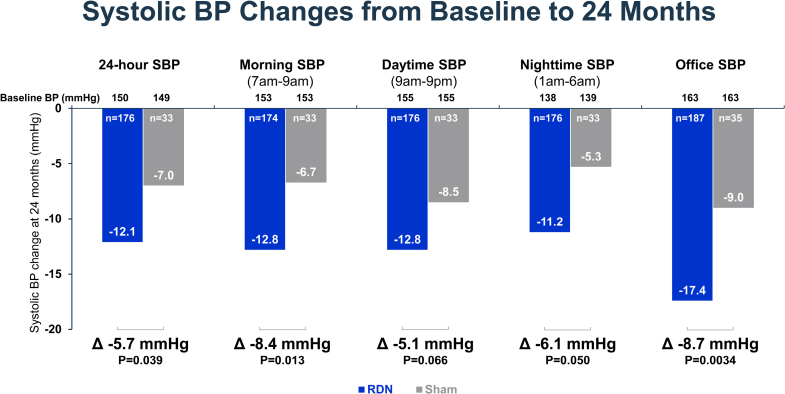
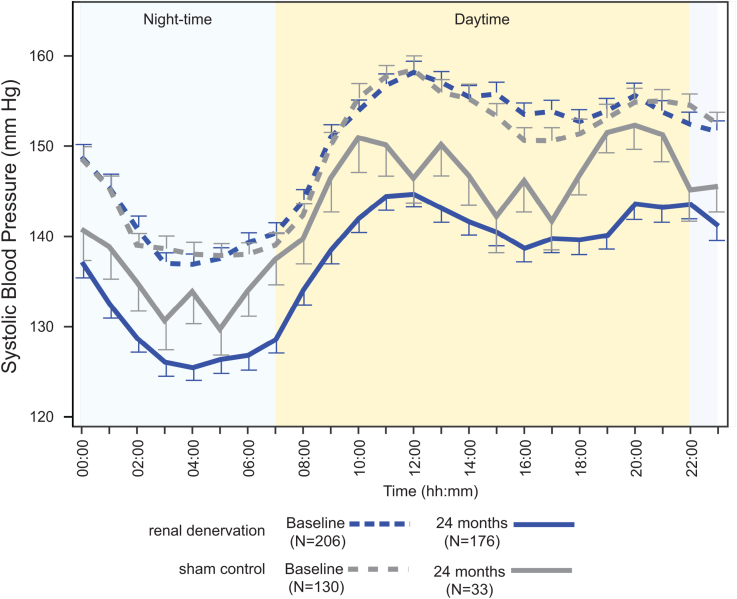
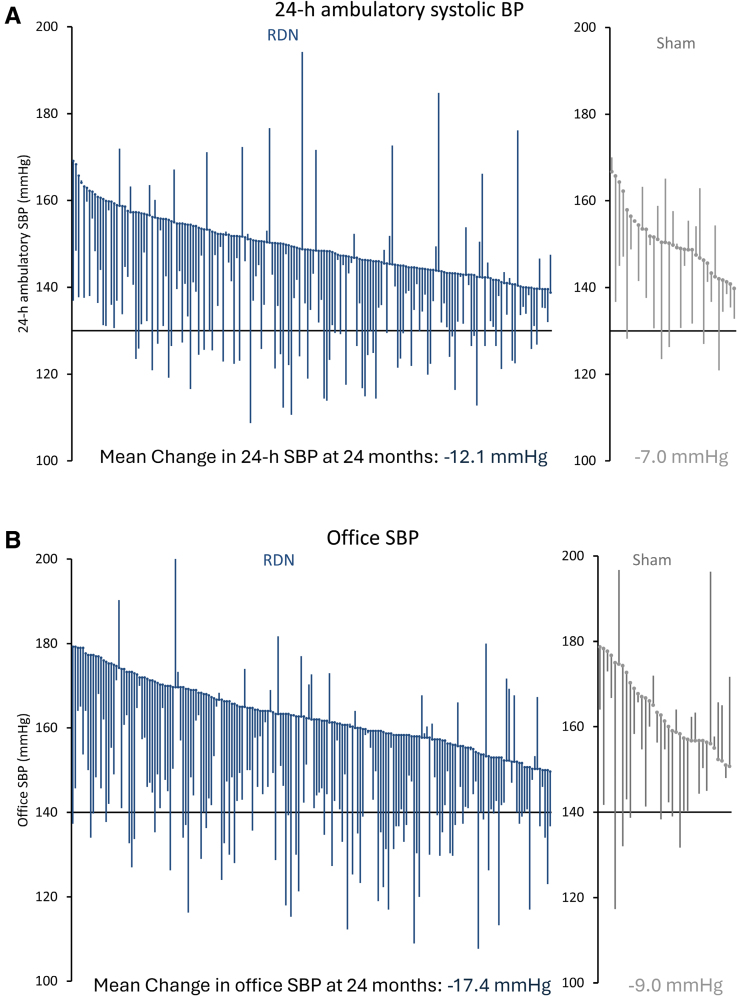
At 24 months, the RDN group demonstrated a significantly greater mean reduction in 24-hour ambulatory systolic blood pressure compared to sham controls: -12.1 ± 15.3 mm Hg (n=176) versus -7.0 ± 13.1 mm Hg (n=33), with a mean difference of -5.7 mm Hg (P=0.039). Office systolic blood pressure reductions were also significantly greater in the RDN group: -17.4 ± 16.1 mm Hg (n=187) versus -9.0 ± 19.4 mm Hg (n=35), mean difference -8.7 mm Hg (P=0.0034).
Despite these differences, antihypertensive medication use increased more in the sham group over 24 months (from 1.7 to 2.7 medications) than in the RDN group (from 1.8 to 2.4 medications; P=0.046). This suggests the blood pressure benefit in RDN was achieved with less up-titration of pharmacotherapy, indicating a genuine procedural effect independent of medication escalation.
Sensitivity analyses that accounted for missing BP values in crossover patients reinforced the findings, showing consistent blood pressure reductions favoring RDN (ambulatory systolic P=0.023; office systolic P<0.0001).
Regarding safety, clinically relevant adverse events were rare, with no cases of renal artery stenosis detected during follow-up. This affirms the favorable long-term safety profile of the RDN procedure.
Expert Commentary
The SPYRAL HTN-ON MED 24-month results provide robust evidence supporting the sustained efficacy and safety of catheter-based renal denervation as an adjunctive treatment for hypertension. The durability of blood pressure reductions despite unblinding and medication adjustments reinforces the physiological plausibility that RDN disrupts sympathetic renal nerve input, contributing to long-lasting blood pressure control.
While the modest absolute differences in BP reduction may seem small, even a 5–8 mm Hg systolic reduction can translate into substantial cardiovascular risk reduction at the population level. This is especially relevant for patients with uncontrolled hypertension despite pharmacotherapy, a group that often experiences poor outcomes.
Limitations include the crossover design after 6 months, which reduces the sham group size at 24 months and may introduce bias despite statistical handling. Moreover, longer-term data beyond 24 months are necessary to assess durability further and clinical outcome impacts such as stroke or myocardial infarction. Finally, the trial population mainly included patients on 1–3 medications; thus, the generalizability to patients with more resistant hypertension or broader demographics requires further research.
Conclusion
The SPYRAL HTN-ON MED trial demonstrates that renal denervation achieves sustained and significant reductions in ambulatory and office systolic blood pressure over 24 months with good safety and less dependence on increasing antihypertensive medications compared with sham controls. These findings support the role of RDN as an effective, long-term complementary therapy for patients with hypertension receiving standard medical treatment, potentially improving cardiovascular risk profiles.
Further studies are needed to clarify patient selection criteria, long-term clinical outcomes, and integration with evolving hypertension management paradigms.
References
1. Kandzari DE, Mahfoud F, Townsend RR, Kario K, Weber MA, Schmieder RE, Tsioufis K, Pocock S, Liu M, DeBruin V, Brar S, Böhm M. Long-Term Safety and Efficacy of Renal Denervation: 24-Month Results From the SPYRAL HTN-ON MED Trial. Circ Cardiovasc Interv. 2025 Jul;18(7):e015194. doi: 10.1161/CIRCINTERVENTIONS.125.015194 IF: 7.4 Q1 . Epub 2025 May 20. PMID: 40391448 IF: 7.4 Q1 ; PMCID: PMC12244969 IF: 7.4 Q1 .