Highlight
– The PRAETORIAN-XL trial provides the longest follow-up comparison (median 87.5 months) between subcutaneous ICD (S-ICD) and transvenous ICD (TV-ICD) regarding device-related complications.
– No significant difference was observed in the overall incidence of device-related complications.
– TV-ICDs were associated with significantly higher rates of major complications and lead-related issues.
– S-ICD should be considered a preferred option in patients without pacing indications.
Study Background and Disease Burden
Implantable cardioverter defibrillators (ICDs) are considered standard life-saving devices for the prevention of sudden cardiac death in patients at high risk for malignant ventricular arrhythmias. Traditionally, transvenous ICDs (TV-ICDs) have been the mainstay of therapy; however, they carry risks particularly linked to the intravascular leads, including lead fracture, infection, and venous thrombosis. These complications often manifest after long-term follow-up and may require invasive interventions.
The subcutaneous ICD (S-ICD) was developed to mitigate lead-related risks by avoiding transvenous lead implantation. Early data from the PRAETORIAN trial demonstrated noninferiority of the S-ICD compared to TV-ICD concerning combined endpoints of device-related complications and inappropriate shocks at approximately 4 years. However, because lead-related complications with TV-ICDs tend to accumulate over time, a longer follow-up period is essential to understand the true comparative safety profile.
The PRAETORIAN-XL trial extends this investigation to an 8-year horizon, aiming to determine whether the safety advantages of S-ICD become more pronounced over long-term follow-up.
Study Design
The PRAETORIAN trial was a multicenter, prospective, randomized controlled trial conducted at 39 centers across the United States and Europe from March 2011 to January 2017. Patients with a class I or IIa indication for ICD therapy without pacing requirements were randomized to receive either S-ICD or TV-ICD.
The PRAETORIAN-XL study represents an extension of the original trial, adding approximately 4 additional years of follow-up beyond the initial median 49.1 months for a total median follow-up of 87.5 months (about 7.3 years).
The primary endpoint was a composite of all device-related complications, including those related and unrelated to leads, categorized as minor or major, with major complications defined as those requiring invasive interventions. Statistical analysis employed a modified intention-to-treat approach using Fine-Gray subdistribution hazard models accounting for competing risks. An as-treated analysis with Cox proportional hazards models included device type as a time-dependent variable to reflect changes over time.
Key Findings
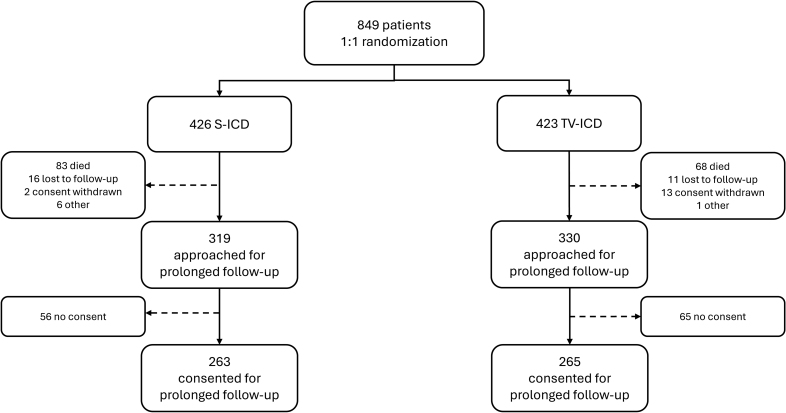
A total of 849 patients were randomized (426 to S-ICD and 423 to TV-ICD). Patients’ median age ranged from 63 to 64 years, with female representation at 21% in the S-ICD group and 18% in the TV-ICD group.
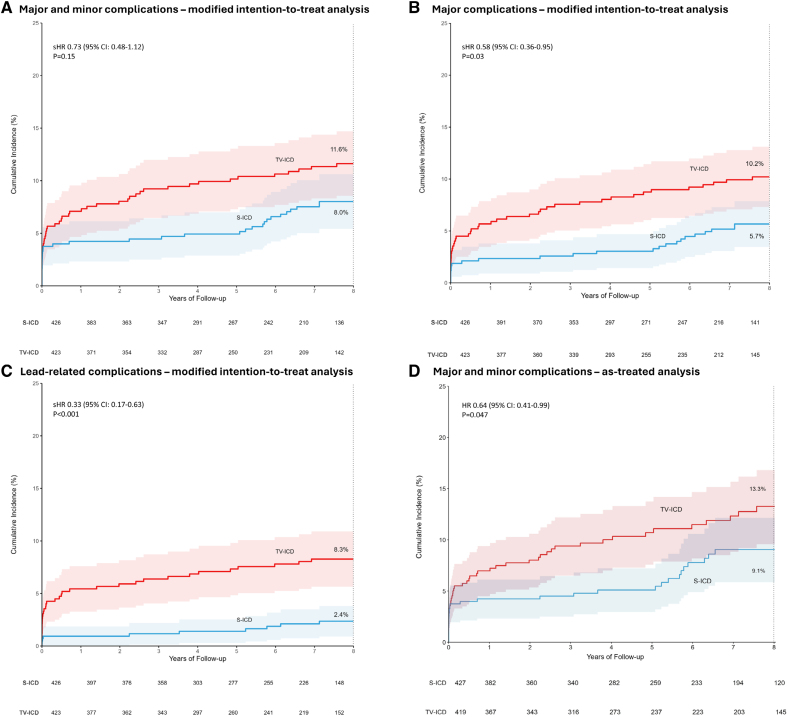
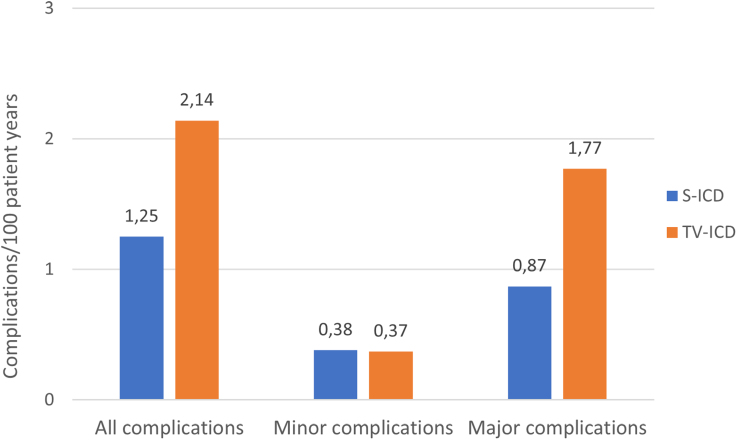
After a median of 87.5 months, the modified intention-to-treat analysis showed no statistically significant difference in the composite endpoint of all device-related complications between the two groups (subdistribution hazard ratio [HR] 0.73; 95% confidence interval [CI], 0.48–1.12; P=0.15).
Eight-year estimated cumulative incidences of device-related complications
However, TV-ICD patients experienced significantly more major complications (P=0.03) and lead-related complications (P<0.001) than did patients with S-ICDs. The as-treated analysis confirmed that patients with TV-ICDs had a higher overall complication rate compared with those with S-ICDs (HR 0.64; 95% CI, 0.41–0.99; P=0.047).
These findings emphasize that while the aggregate complication rates may appear similar, the nature and severity of those complications differ, with TV-ICD complications having potentially greater clinical impact due to invasiveness and lead involvement.
Expert Commentary
The PRAETORIAN-XL trial robustly confirms that lead-related complications remain a significant concern for TV-ICD recipients during extended follow-up. These complications can include lead fracture, insulation breaches, and infections, which often require complex lead extraction procedures with associated morbidity and mortality risks.
Subcutaneous ICDs offer a compelling alternative by avoiding transvenous leads, thus inherently reducing risks of intravascular complications. However, S-ICDs lack pacing capabilities, including anti-tachycardia pacing and bradycardia support, limiting use to patients without pacing indications.
Clinical guidelines increasingly acknowledge the S-ICD as a valuable option in appropriate patients. The PRAETORIAN-XL data support this perspective, suggesting S-ICDs can enhance long-term safety without compromising efficacy.
Limitations of the study include the evolving ICD technology and implantation techniques since the study inception and the exclusion of patients requiring pacing, limiting generalizability. Real-world registries and further research are needed to fully define patient selection criteria and long-term comparative effectiveness.
Conclusion
Over an extended median follow-up of more than 7 years, the PRAETORIAN-XL trial demonstrates that subcutaneous ICDs have a more favorable profile regarding major and lead-related complications compared to transvenous ICDs. While overall device-related complication rates are statistically comparable, the risk profile favors S-ICDs due to their design advantages.
Clinicians should consider S-ICD therapy preferentially for patients without pacing indications to reduce long-term procedural risks and improve patient safety. This paradigm shift aligns with precision medicine goals to tailor device therapy according to individual risk-benefit profiles.
References
Olde Nordkamp LRA, de Veld JA, Ghani A, et al; PRAETORIAN-XL Investigators. Device-Related Complications in Transvenous Versus Subcutaneous Defibrillator Therapy During Long-Term Follow-Up: The PRAETORIAN-XL Trial. Circulation. 2025 Jul 22;152(3):172-182. doi: 10.1161/CIRCULATIONAHA.125.074576 IF: 38.6 Q1 . Epub 2025 Apr 25. PMID: 40279654 IF: 38.6 Q1 ; PMCID: PMC12272918 IF: 38.6 Q1 .
National Institute for Health and Care Excellence (NICE). Implantable cardioverter defibrillators. NICE guideline [NG148]. 2019.
Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities. Circulation. 2008;117(21):e350-e408.