Highlights
- The MiniMed 780G automated insulin delivery (AID) system significantly improved time in range (TIR) and HbA1c compared to manual insulin pump therapy in children aged 2–6 years.
- Safety profile was acceptable, with no severe hypoglycaemia and a single diabetic ketoacidosis event unrelated to device malfunction.
- The LENNY trial supports early adoption of AID to potentially prevent long-term diabetes complications in young children.
Clinical Background and Disease Burden
Type 1 diabetes mellitus (T1DM) in early childhood presents profound challenges due to the dynamic physiology, unpredictable dietary intake, and high vulnerability to hypoglycaemia. In children aged 2–6 years, glycaemic instability is common and can negatively impact cognitive development and neuroplasticity. Achieving optimal glycaemic control in this population is complicated by behavioral, developmental, and physiological factors, leading to an unmet need for safer and more effective insulin delivery approaches. Automated insulin delivery (AID) systems, which integrate glucose monitoring with algorithm-driven insulin dosing, offer a promising strategy to address this clinical gap.
Research Methodology
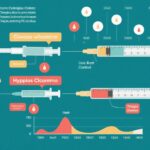
The LENNY trial was an open-label, multicentre, randomized, crossover study conducted across 12 hospitals in Finland, Italy, Slovenia, and the UK. It enrolled 98 children aged 2–6 years with T1DM requiring at least 6 units of insulin per day. After a 2-week run-in with the MiniMed 780G system in manual mode plus suspend before low (SBL), participants were randomly assigned to one of two sequences: 12 weeks of auto mode followed by 12 weeks of manual+SBL mode (separated by a 2-week washout), or the reverse. The primary endpoint was the adjusted between-treatment difference in the percentage of TIR (70–180 mg/dL), with a non-inferiority margin of 7.5 percentage points. Secondary endpoints included differences in mean HbA1c and assessment for superiority in both TIR and HbA1c. Safety was monitored throughout.
Key Findings
A total of 98 children (mean age 4.7 years) completed the study phase, with balanced representation by sex and robust geographic diversity. The TIR improved from 58.1% during the run-in to 68.3% in auto mode, compared to 58.3% in manual+SBL mode. The adjusted between-treatment difference was 9.9 percentage points (95% CI 8.0–11.7), meeting the predefined criteria for non-inferiority and demonstrating clear superiority for the auto mode.
Similarly, mean HbA1c improved from 7.53% at baseline to 7.00% in auto mode, versus 7.61% in manual+SBL mode. The adjusted difference was –0.61 percentage points (95% CI –0.76 to –0.46), again meeting and exceeding non-inferiority and showing superiority for auto mode. Notably, no episodes of severe hypoglycaemia occurred during either treatment arm. Nine serious adverse events were reported, with only one case of diabetic ketoacidosis (DKA) during auto mode, which was unrelated to device malfunction or trial procedures.
Mechanistic Insights
The MiniMed 780G system leverages continuous glucose monitoring and adaptive algorithms to modulate insulin delivery in real-time, thereby reducing glycaemic excursions. In young children, this dynamic adjustment is particularly valuable, given their variable insulin sensitivity and unpredictable carbohydrate intake. The improved TIR and HbA1c observed in the LENNY trial are consistent with the expected physiological benefits of algorithm-driven therapy, minimizing both hyperglycaemia and hypoglycaemia.
Expert Commentary
The LENNY trial represents a pivotal advance in paediatric diabetes management. Recent guidelines from the International Society for Pediatric and Adolescent Diabetes (ISPAD) and the American Diabetes Association emphasize the importance of achieving near-normal glycaemic targets without increasing hypoglycaemia risk. The robust improvements in TIR and HbA1c, without a safety trade-off, position AID systems as a potential new standard for young children. Dr. Tadej Battelino and colleagues highlight that these findings may have long-term implications for neurodevelopmental outcomes and overall diabetes prognosis in this vulnerable age group.
Controversies or Limitations
Despite its strengths, the LENNY trial has several limitations. The open-label design could introduce bias, although objective endpoints such as TIR and HbA1c mitigate this concern. The relatively short duration (12 weeks per treatment arm) may not capture long-term adherence or rare adverse events. Furthermore, all participating families were able to use advanced diabetes technology, which may limit the generalizability to less-resourced settings. The study was funded by Medtronic, the device manufacturer, which necessitates careful interpretation of the results, though no device-related adverse events were reported.
Conclusion
The LENNY trial demonstrates that the MiniMed 780G AID system delivers superior glycaemic control and an acceptable safety profile in children aged 2–6 years with type 1 diabetes. These findings support the consideration of early AID adoption to improve immediate glycaemic outcomes and potentially mitigate long-term complications. Future research should address longer-term outcomes, cost-effectiveness, and strategies to enhance accessibility across diverse healthcare settings.
References
- Battelino T, Kuusela S, Shetty A, Rabbone I, Cherubini V, Campbell F, et al.; LENNY study group. Efficacy and safety of automated insulin delivery in children aged 2–6 years (LENNY): an open-label, multicentre, randomised, crossover trial. Lancet Diabetes Endocrinol. 2025 Aug;13(8):662–673. doi:10.1016/S2213-8587(25)00091-9. PMID: 40544853.
- International Society for Pediatric and Adolescent Diabetes (ISPAD) Clinical Practice Consensus Guidelines 2022: https://www.ispad.org/page/ISPADGuidelines2022
- American Diabetes Association. 14. Children and Adolescents: Standards of Medical Care in Diabetes—2024. Diabetes Care. 2024;47(Supplement_1):S237–S249. doi:10.2337/dc24-S014