Highlight
– Exposure to certain antibiotics (excluding macrolides and penicillins) correlates with decreased overall survival (OS) in advanced NSCLC patients receiving pembrolizumab.
– Steroid use at doses above 20 mg prednisone equivalent for pembrolizumab monotherapy and above 30 mg combined with chemotherapy is linked to poorer survival.
– Proton pump inhibitors (PPIs) are independently associated with worse OS in this patient population.
– These findings emphasize the need for careful evaluation of comedications to optimize immunotherapy outcomes.
Study Background and Disease Burden
Non-small-cell lung cancer (NSCLC) represents the most common type of lung cancer and is a major cause of cancer-related mortality worldwide. The advent of immune checkpoint inhibitors, such as pembrolizumab, a programmed death 1 (PD-1) inhibitor, has transformed first-line therapy for advanced NSCLC, improving survival outcomes. Nevertheless, treatment efficacy varies substantially among patients, influenced not only by tumor molecular characteristics but also by host factors, including concomitant medications.
Antibiotics, systemic corticosteroids, and proton pump inhibitors (PPIs) are frequently administered to cancer patients for various comorbid conditions or cancer-related symptoms. Emerging evidence suggests these medications may negatively impact the efficacy of immune checkpoint inhibitors by altering the gut microbiome, modulating immune responses, or affecting drug metabolism. However, robust population-scale data examining their association with survival outcomes in pembrolizumab-treated advanced NSCLC patients are limited.
Study Design
In this large-scale, nationwide retrospective cohort study, researchers used target trial emulations leveraging the French national health care database to examine the effect of concomitant medications on overall survival in advanced NSCLC. Patients newly diagnosed from January 2015 through December 2022 who initiated first-line pembrolizumab and were alive two months post-treatment initiation were included.
Exclusion criteria ruled out patients hospitalized for infectious diseases, autoimmune disorders, or peptic ulcer disease to avoid confounding by indication for the studied comedications.
Medication exposures were defined as follows: Antibiotic and PPI exposure required at least two prescriptions from 60 days before to 42 days after the pembrolizumab start date; steroid exposure required at least two prescriptions from 30 days before to 30 days after pembrolizumab initiation. Steroid dose intensity was also evaluated based on prednisone-equivalent daily dosage.
The primary endpoint was overall survival (OS). An inverse probability of treatment weighting (IPTW) analytic approach was used to minimize confounding bias when comparing outcomes between exposed and unexposed patients.
Key Findings
The study population comprised 41,529 advanced NSCLC patients treated with first-line pembrolizumab: 27,826 males (67%), median age 65 years (range 19–97). Among them, 35.7% received pembrolizumab monotherapy, whereas 64.3% received pembrolizumab combined with chemotherapy.
Figure 1. Weighted Distribution by Antibiotc, Steroid, or Proton Pump Inhibitor (PPI) Exposure.

At treatment initiation, 41.9% were exposed to antibiotics, 59.1% to steroids, and 53.7% to PPIs.
Antibiotics: After IPTW adjustment, antibiotic use was associated with a modest but statistically significant reduction in OS (hazard ratio [HR] 1.08, 95% confidence interval [CI], 1.05–1.12; P < .001). However, this association was not uniform across all antibiotic classes—with macrolides and penicillins not showing adverse effects on survival, suggesting heterogeneity by antibiotic type potentially linked to differential microbiome disruption or immune modulation.
Figure 2. Assocications of Overall Survival (OS) With Antibiotic Exposure Among Those Receiving Pembrolizumab Alone and Pembrolizumab Plus Chemotherapy.

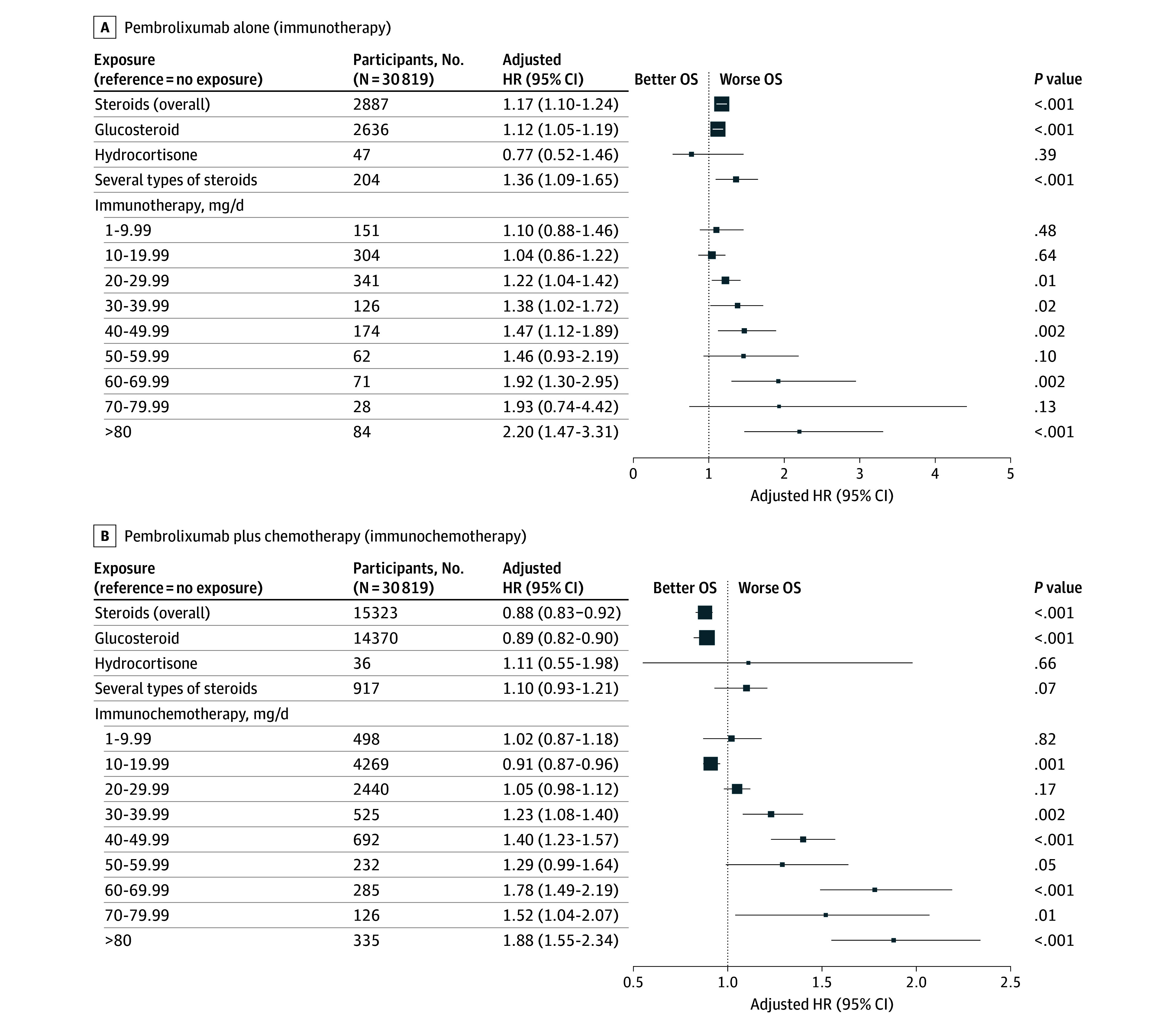
Steroids: Overall steroid exposure showed no significant association with OS (HR 0.98, 95% CI 0.95–1.02; P = .37). Yet, a significant interaction was found with the pembrolizumab regimen (monotherapy versus combination chemotherapy) (P < .001). Dose-dependent analysis revealed worsening survival with higher prednisone-equivalent doses: >20 mg/day associate with poor outcomes in monotherapy patients (P for trend = .005), and >30 mg/day in combination therapy patients (P for trend < .001). This points to the immunosuppressive effects of higher steroid doses impacting anti-tumor immunity during immunotherapy.
Figure 3. Associations of Overall Survival (OS) With Steroid Exposure Among Those Receiving Pembrolizumab Alone and Pembrolizumab Plus Chemotherapy.
Proton Pump Inhibitors (PPIs): PPI use was associated with significantly worse OS (HR 1.13, 95% CI 1.10–1.17; P < .001), consistent with previous reports implicating PPIs in disrupting gut microbiota and impairing immune checkpoint inhibitor efficacy.
Figure 4. Proton Pump Inhibitor (PPI) Exposure and Overall Survival (OS).
Expert Commentary
This large real-world analysis extends prior smaller studies linking concomitant medications to immunotherapy outcomes, providing granular insights into class-specific and dose-dependent effects. The observed antibiotic-class effects align with the hypothesis that microbiome perturbations influence immunotherapy efficacy. Steroid dosing thresholds identified help refine clinical decisions balancing symptom control and immune preservation.
Nevertheless, as a retrospective observational study, residual confounding is possible despite sophisticated IPTW adjustments. Confounding by indication cannot be entirely excluded — patients requiring these medications may have inherently worse prognosis or more aggressive disease manifestations. The study’s exclusion of certain comorbid conditions mitigates but does not eliminate this risk.
Mechanistically, antibiotics and PPIs may impair beneficial commensal gut bacteria essential for priming anti-tumor T cell responses. High-dose steroids blunt T-cell function and cytokine production vital for checkpoint blockade mechanisms. Future prospective and mechanistic investigations are essential to fully delineate causality.
Conclusion
This comprehensive nationwide cohort study highlights that certain antibiotics, high-dose steroids, and PPIs concurrent with first-line pembrolizumab treatment in advanced NSCLC are linked to reduced overall survival. These findings emphasize the critical need for judicious prescribing and close monitoring of comedications in patients undergoing immunotherapy.
Clinicians should carefully assess the indication, selection, and dosing of antibiotics and steroids, and reconsider PPI use when possible to optimize immunotherapy outcomes. These results add to the growing evidence that host and microbiome modulation via concomitant medications significantly influence immune checkpoint inhibitor effectiveness.
Ongoing research should integrate microbiome profiling, immune phenotyping, and prospective intervention trials to develop tailored strategies mitigating negative comedication effects and enhancing personalized cancer immunotherapy.
References
Rousseau A, Simon-Tillaux N, Michiels S, et al. Concomitant Comedications and Survival With First-Line Pembrolizumab in Advanced Non-Small-Cell Lung Cancer. JAMA Netw Open. 2025;8(9):e2529225. doi:10.1001/jamanetworkopen.2025.29225 IF: 9.7 Q1 .
Derosa L, Routy B, Kroemer G, Zitvogel L. Microbiota and immunotherapy: diagnostic and therapeutic potential. Gastroenterology. 2021;160(7):2023-2041.
Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36(28):2872-2878.
Pinato DJ, Howlett S, Ottaviani D, et al. Association of prior antibiotic use with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 2019;5(12):1774-1778.