Highlight
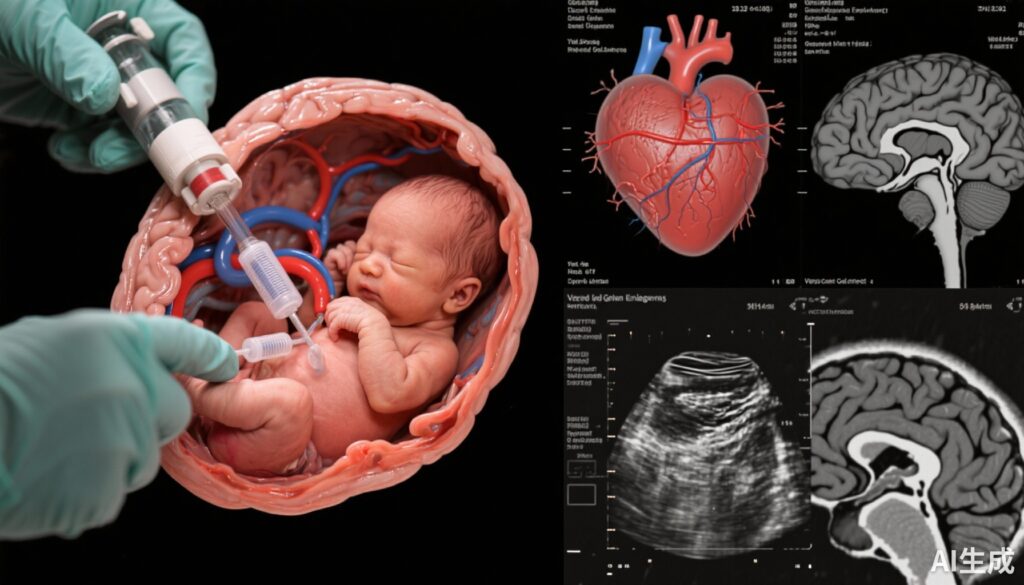
• Vein of Galen malformation (VOGM) is a serious congenital cerebrovascular defect with high neonatal mortality and neurodevelopmental risk.
• This pioneering single-center study reports early clinical outcomes of in utero embolization in fetuses with high-risk VOGM.
• In utero embolization reduced fetal cardiac output and showed a trend toward improved survival and neurodevelopmental outcomes in treated fetuses.
• However, fetal intervention was associated with a high rate of unscheduled and preterm deliveries, posing new clinical challenges.
Study Background and Disease Burden
Vein of Galen malformation (VOGM) represents the most common congenital cerebrovascular anomaly characterized by abnormal arteriovenous shunting into the median prosencephalic vein of Markowski. This results in enlarged venous structures and high-volume shunts that can overwhelm cardiovascular and cerebral physiology. In neonates, VOGM may lead to severe congestive heart failure, neurologic injury, and neurodevelopmental delays. Mortality rates are notably high for fetuses with concomitant wide mediolateral falcine sinus diameters, which predict compromised brain maturation and poor postnatal milestones. Conventional management focuses on postnatal embolization, but neonatal stability often limits therapeutic success. Hence, innovation in fetal intervention offers a potentially transformative approach to mitigate early pathophysiology and improve survival and functional outcomes.
Study Design
This prospective single-group intervention study was conducted at a US tertiary center with institutional review board approval. Enrollment started on September 30, 2022, with follow-up through April 10, 2025. Inclusion criteria were fetuses diagnosed with VOGM confirmed by fetal magnetic resonance imaging (MRI), absence of major brain injury, and a falcine sinus diameter ≥7 mm, a critical marker of high risk. The intervention consisted of ultrasound-guided in utero embolization using transuterine, transcranial access to microcatheterize the prosencephalic venous varix. Detachable coils were deployed to reduce shunting. Main outcome measures included neonatal mortality and standardized neurodevelopmental outcomes assessed at six months, supplemented by pre- and postembolization fetal MRI and echocardiography to monitor morphological and hemodynamic changes.
Key Findings
Seven pregnant patients with fetal VOGM were enrolled: maternal age ranged from 22 to 36 years (mean 32.4), fetal gestational age at intervention ranged from 33 6/7 to 37 1/7 weeks (mean 35 6/7), with a female:male sex ratio of 3:4. The mean falcine sinus width was 10.3 mm, correlated historically with a 90% predicted mortality and only 9% chance of achieving six-month developmental milestones under standard postnatal care.
Of the seven, five fetuses underwent successful embolization. The procedure was associated with a significant mean reduction in cardiac output of 33.4% (range 16%-46%), suggesting effective shunt volume reduction and potential improvement in cardiac load. Mortality among the cohort was 43%. Notably, 43% of survivors met expected neurodevelopmental milestones at six-month follow-up. Three embolized patients survived beyond infancy (8, 18, and 24 months) without neurodevelopmental delay, signaling potential long-term benefit.
Postnatal interventions included additional neonatal embolizations in four infants, demonstrating the necessity of a staged approach. However, fetal intervention was complicated by a high incidence of unscheduled deliveries (71.4%), three of which were preterm, occurring a mean of 3.2 days post-procedure, highlighting risk tradeoffs.
Expert Commentary
The feasibility of fetal embolization for VOGM demonstrated by Orbach et al. represents a significant advance in prenatal management of high-risk cerebrovascular anomalies. This study fills a critical gap by addressing prenatal hemodynamic compromise and may fundamentally shift paradigm from reactive postnatal care to proactive fetal intervention. The marked reduction in cardiac output post-embolization provides mechanistic plausibility for improved cardiac and cerebral physiology.
However, clinical translation is tempered by notable risks, chiefly preterm labor and delivery, which introduce morbidity risks associated with prematurity. Safety profiles will require elaboration in larger cohorts. Furthermore, long-term developmental follow-up beyond infancy is essential to confirm sustained benefit. The study also raises questions on optimal timing of intervention, selection criteria refinement, and procedural technicalities to minimize complications.
Current guidelines do not yet incorporate fetal embolization, signaling an area for expert consensus and future multicenter trials. For clinicians counseling families, these early data allow balanced information on potential survival and neurodevelopmental gains versus obstetrical risks.
Conclusion
In utero embolization for fetal Vein of Galen malformation emerges as a technically feasible and potentially impactful intervention that could reduce mortality and enhance neurodevelopmental trajectories in a traditionally high-risk group. Nevertheless, the benefits must be balanced against a significant risk of preterm and unscheduled delivery, underscoring the need for careful patient selection, multidisciplinary care, and further research to optimize outcomes. This pioneering approach heralds a new front in fetal therapy with promising implications for congenital cerebrovascular disease management.
References
1. Orbach DB, Shamshirsaz AA, Wilkins-Haug L, et al. In Utero Embolization for Fetal Vein of Galen Malformation. JAMA. 2025;334(10):878-885. doi:10.1001/jama.2025.12363
2. McElhinney DB, Tworetzky W. Vein of Galen malformations: state of the art. Pediatr Cardiol. 2012;33(3):223-232. doi:10.1007/s00246-011-0147-3
3. Steinlin M, Boltshauser E. Vein of Galen malformations. Eur J Paediatr Neurol. 2012;16(3):242-254. doi:10.1016/j.ejpn.2011.11.001
4. Lasjaunias P, Terbrugge KG, Berenstein A. Surgical neuroangiography. Vol. 1. Clinical vascular anatomy and variations. Berlin: Springer-Verlag; 2006.