Highlight
Dual immune checkpoint blockade with ipilimumab and nivolumab failed to improve progression-free survival compared to standard temozolomide chemotherapy in MGMT-unmethylated newly diagnosed glioblastoma. The phase II trial did not meet its primary endpoint, resulting in early termination without proceeding to phase III. Safety profiles were consistent with known effects, and no new adverse signals emerged.
Molecular correlative studies and ongoing survival follow-up may inform future therapeutic strategies in this difficult-to-treat subgroup.
Study Background and Disease Burden
Glioblastoma (GBM) remains the most common and aggressive primary brain malignancy in adults, characterized by poor prognosis despite multimodal treatment. The current standard of care involves maximal safe surgical resection followed by radiotherapy combined with temozolomide (TMZ) chemotherapy. However, patients harboring glioblastomas with unmethylated O6-methylguanine-DNA methyltransferase (MGMT) promoter status (‘uMGMT’) derive significantly less benefit from TMZ due to intrinsic resistance. This subgroup faces notably shorter progression-free survival (PFS) and overall survival (OS), highlighting a critical unmet need for novel therapies.
Immune checkpoint inhibitors targeting CTLA-4 (ipilimumab) and PD-1 (nivolumab) have transformed treatment paradigms in several malignancies but have yet to demonstrate clear benefit in glioblastoma. Earlier phase I investigations including the NRG Oncology BN002 trial established the safety and suggested potential signals of efficacy for combined ipilimumab and nivolumab in newly diagnosed GBM. This rationale spurred the design of the NRG Oncology BN007 randomized Phase II/III trial targeted at the uMGMT GBM population, testing whether dual immune checkpoint blockade could improve outcomes over standard TMZ.
Study Design
The NRG Oncology BN007 was a randomized, controlled, open-label Phase II/III clinical trial enrolling adults with newly diagnosed, histologically confirmed MGMT-unmethylated glioblastoma. Eligible patients had a Karnofsky performance status (KPS) ≥ 70 and had undergone maximal safe resection.
Participants were randomized 1:1 to receive radiotherapy combined either with immunotherapy (ipilimumab plus nivolumab) or with standard temozolomide chemotherapy. Stratification factors included recursive partitioning analysis (RPA) class (III, IV, V) and planned use of tumor treating fields.
Key protocol features included disallowing corticosteroids at immunotherapy initiation to minimize immunosuppression and centralized confirmation of diagnosis, biomarker status (MGMT methylation), and PFS events. The primary endpoint for Phase II was progression-free survival (PFS), with the study powered (95%) to detect a hazard ratio (HR) ≤ 0.58 favoring immunotherapy at a one-sided significance level of 0.15. Success in Phase II PFS would trigger continuation into Phase III overall survival (OS) analysis.
Key Findings
Between enrollment completion and preplanned interim analysis, 159 patients were randomized: 79 to the immunotherapy arm and 80 to the TMZ arm. Baseline demographics and clinical features were well balanced, with median age 60 years, 66% male, the majority (61%) having KPS 90-100, and 65% achieving gross total resection. Distribution of RPA classes was predominantly class IV (73%).
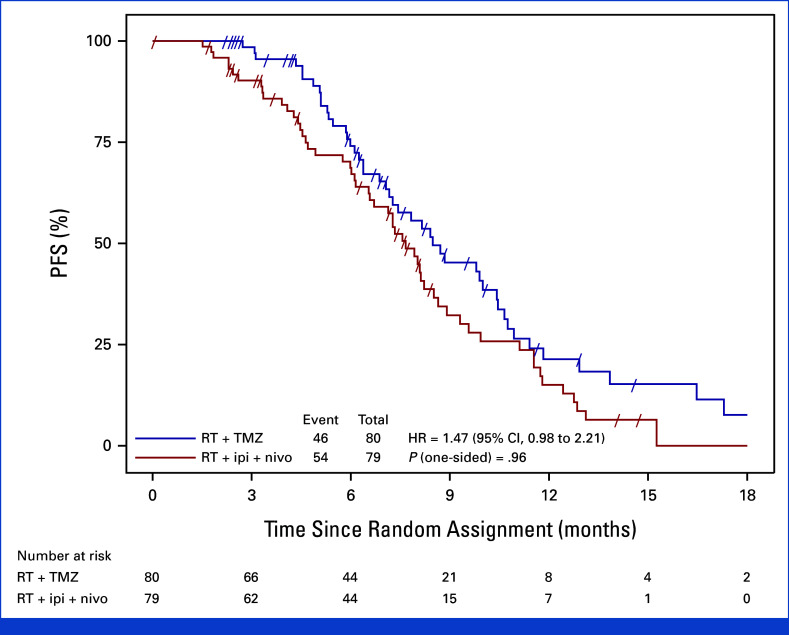
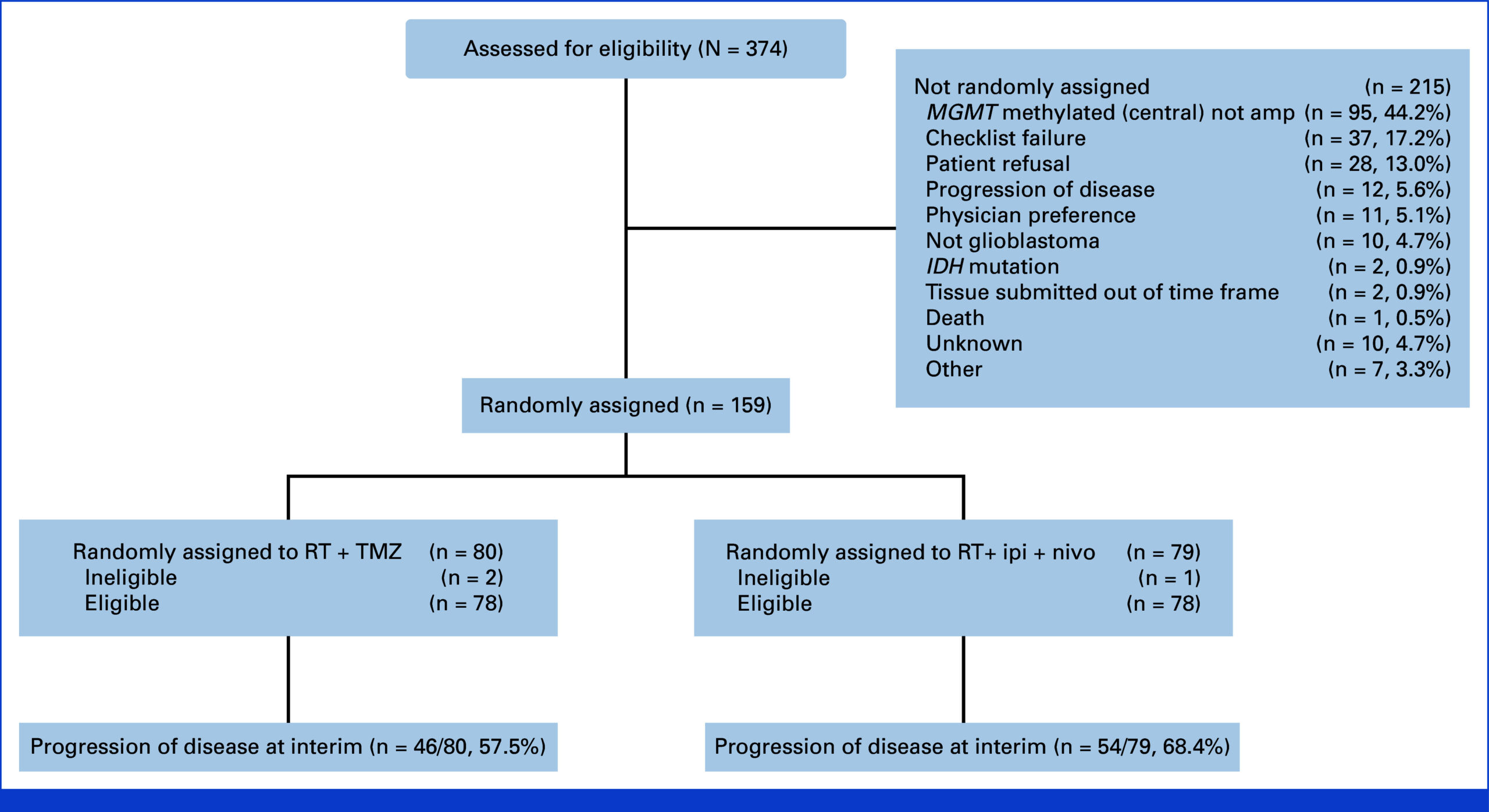 After 100 centrally adjudicated progression events, the interim analysis showed no improvement in PFS with immunotherapy compared to TMZ. Median PFS was 7.7 months in the ipilimumab plus nivolumab group versus 8.5 months in the TMZ group (hazard ratio, 1.47; 70% confidence interval, 1.19 to 1.83). The one-sided P value was 0.96, indicating no statistical significance favoring the experimental arm.
After 100 centrally adjudicated progression events, the interim analysis showed no improvement in PFS with immunotherapy compared to TMZ. Median PFS was 7.7 months in the ipilimumab plus nivolumab group versus 8.5 months in the TMZ group (hazard ratio, 1.47; 70% confidence interval, 1.19 to 1.83). The one-sided P value was 0.96, indicating no statistical significance favoring the experimental arm.
Overall survival data remain immature with more than 50% of participants alive at the time of analysis; median OS was approximately 13 months in both arms (HR, 0.95; 95% CI, 0.61 to 1.49; P = .36), showing no survival advantage with dual immune checkpoint blockade.
 Regarding safety, no new toxicity signals emerged beyond the known adverse event profiles of ipilimumab and nivolumab. Immune-related adverse events were manageable and did not differ markedly from prior reports. Notably, corticosteroids were prohibited at immunotherapy initiation to optimize immune responsiveness.
Regarding safety, no new toxicity signals emerged beyond the known adverse event profiles of ipilimumab and nivolumab. Immune-related adverse events were manageable and did not differ markedly from prior reports. Notably, corticosteroids were prohibited at immunotherapy initiation to optimize immune responsiveness.
Expert Commentary
This trial adds to the growing body of evidence that checkpoint inhibitors, despite their remarkable impact on multiple solid tumors, may have limited efficacy in newly diagnosed glioblastoma, especially in the MGMT-unmethylated subgroup. The microenvironmental factors, blood-brain barrier challenges, and limited T-cell infiltration potentially underpin these disappointing results.
The failure to improve PFS or OS with ipilimumab and nivolumab corroborates similar outcomes from other immunotherapy studies in GBM. The trial’s robust design with central confirmation of molecular status, a well-stratified population, and careful steroid management strengthens confidence in these conclusions.
Limitations include the relatively early interim analysis and immature OS data, although the PFS hazard ratio strongly argued against immunotherapy benefit. Future research should explore combination approaches that enhance immune infiltration or target additional tumor vulnerabilities alongside checkpoint inhibition.
Conclusion
The NRG Oncology BN007 randomized Phase II trial showed that combining ipilimumab with nivolumab does not improve progression-free survival over temozolomide in patients with newly diagnosed, MGMT-unmethylated glioblastoma undergoing radiotherapy. Consequently, the trial was permanently closed before progressing to Phase III OS analysis.
These findings underscore the ongoing challenge of advancing immunotherapy in glioblastoma and the pressing need for alternative strategies in this patient subgroup. Ongoing molecular and correlative studies from this trial may yield insights that inform future personalized immunotherapeutic approaches.
References
1.Lassman AB, Polley MC, Iwamoto FM, Sloan AE, Wang TJC, Aldape KD, Wefel JS, Gondi V, Gutierrez AN, Manasawala MH, Gilbert MR, Sulman EP, Wolchok JD, Green RM, Neil EC, Lukas RV, Goldlust SA, Snuderl M, Galbraith K, Dignam JJ, Won M, Mehta MP. Dual Immune Check Point Blockade in MGMT-Unmethylated Newly Diagnosed Glioblastoma: NRG Oncology BN007, a Randomized Phase II/III Clinical Trial. J Clin Oncol. 2025 Sep 20;43(27):3032-3040. doi: 10.1200/JCO-25-00618 IF: 41.9 Q1 . Epub 2025 Aug 8. PMID: 40779733 IF: 41.9 Q1 ; PMCID: PMC12440284 IF: 41.9 Q1 .
2. Stupp R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-96.
3. Reardon DA, et al. Immunotherapy advances for glioblastoma. Neuro Oncol. 2020;22(4):555-565.
4. Clarke JL, et al. The role of immune checkpoint blockade in glioblastoma: mechanisms and clinical outcomes. Nat Rev Clin Oncol. 2021;18(4):234-246.