Highlight
- AI digital pathology (DP) significantly improves inter-pathologist agreement in fibrosis staging, particularly early-stage fibrosis (F0-F2) in MASH.
- Utilization of second harmonic generation/two photon excitation fluorescence (SHG/TPEF) imaging combined with AI quantitative fibrosis (qFibrosis) enhances visualization and objective assessment of fibrosis severity.
- AI assistance increases concordance for clinical trial inclusion/exclusion criteria and evaluation of fibrosis response, potentially reducing adjudication needs and increasing trial power.
Study Background and Disease Burden
Metabolic dysfunction-associated steatohepatitis (MASH), previously referred to as nonalcoholic steatohepatitis (NASH), represents a growing cause of chronic liver disease worldwide. Characterized by hepatic steatosis with inflammation and varying degrees of fibrosis, MASH is strongly associated with obesity, insulin resistance, and metabolic syndrome complications. Fibrosis stage is the single most important histological predictor of adverse clinical outcomes in MASH, influencing prognosis and therapeutic decision-making.
Accurate assessment of liver fibrosis via biopsy remains the clinical gold standard; however, significant intra- and inter-pathologist variability in fibrosis staging complicates patient selection for clinical trials and confounds evaluation of treatment efficacy. This interobserver variability, especially in early fibrosis stages pivotal for trial inclusion, may lead to inconsistent patient enrollment and reduced statistical power in trials.
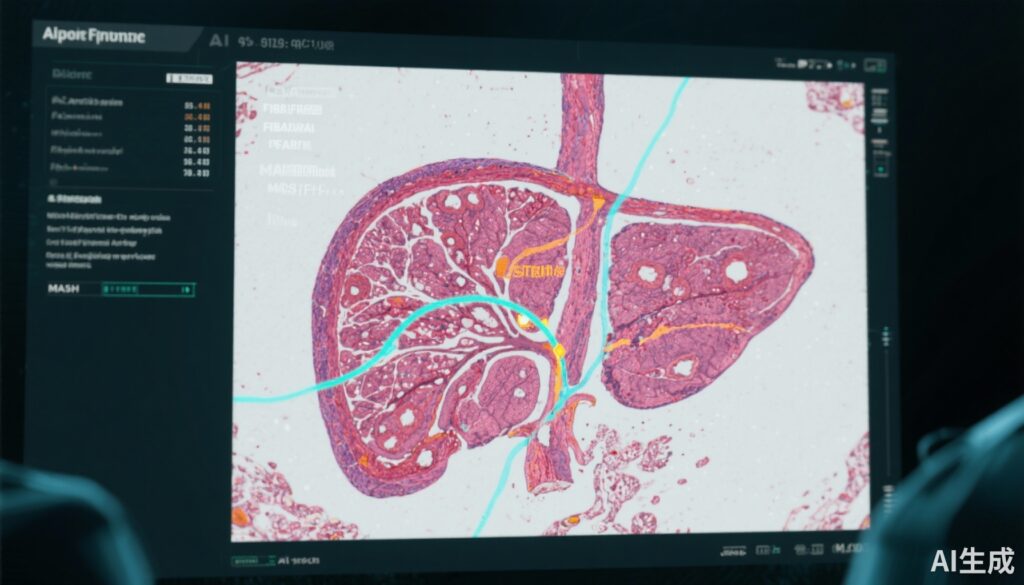
Emerging digital pathology platforms incorporating artificial intelligence (AI) methods aim to augment pathologists’ assessments by providing quantitative and more reproducible fibrosis evaluation tools. This study assessed an AI digital pathology system utilizing SHG/TPEF imaging and AI-driven quantitative fibrosis measures to improve the reliability of fibrosis staging in MASH biopsies.
Study Design
This investigation analyzed 120 digitized liver histology slides sourced from two phase II/III clinical trials registered under identifiers NCT03517540 and NCT03912532. Four expert hepatopathologists independently scored fibrosis stages on these slides both with and without AI assistance, implemented through a randomized, two-period crossover design to control for potential learning or fatigue effects.
The AI digital pathology platform leveraged unstained SHG/TPEF imaging, a label-free modality enabling high-contrast visualization of collagen fibers, combined with AI-derived quantitative fibrosis metrics (qFibrosis). These quantitative values provided continuous fibrosis assessment alongside categorical staging outputs to assist pathologists during scoring.
Primary endpoints included changes in inter- and intra-pathologist agreement as assessed by Cohen’s kappa values. Secondary analyses evaluated concordance related to trial inclusion/exclusion criteria (fibrosis stages F2-F3 for inclusion; F0/F1/F4 for exclusion) and assessment of fibrosis response to treatment.
Key Findings
AI assistance markedly improved inter-pathologist concordance for fibrosis staging, particularly in early fibrosis stages (F0-F2), where variability has traditionally been highest. The inter-rater kappa increased from approximately 0.4 to 0.7 with AI support, a robust improvement indicating substantial agreement. Variance around the median fibrosis stage calls also decreased significantly with AI assistance.
Notably, AI assistance did not alter intra-pathologist consistency, suggesting that individual pathologists’ reliability was stable irrespective of AI aid.
Regarding clinical trial case classification, concordance for inclusion cases (F2-F3) increased from 45% without AI to 71% with AI, while exclusion case concordance improved from 38% to 55%. Similarly, concordance in evaluating fibrosis response to treatment rose from 49% to 61%.
Pathologists assessed the utility of the AI platform components as follows: SHG/TPEF images were found useful in 83% of cases, qFibrosis continuous values in 55%, and qFibrosis staging in 38%, underscoring the value of enhanced visualization and quantitative metrics.
Modeling these improvements in the context of clinical trials suggested about a 25% reduction in the need for adjudication processes and a potential 45% increase in statistical power, enabling smaller sample sizes or more efficient study designs.
Expert Commentary
The use of AI in digital pathology addresses a critical unmet need in MASH clinical research by reducing subjective variability in fibrotic staging. The combination of SHG/TPEF imaging and AI quantitative assessment improves the objectivity and reproducibility of fibrosis evaluation, particularly in early and intermediate stages where detection is challenging.
While intra-pathologist reliability remained unchanged, the enhanced inter-pathologist agreement directly impacts clinical trial robustness by homogenizing patient eligibility determinations and treatment response assessments. This technological augmentation could accelerate drug development timelines and reduce associated costs through more precise histological endpoints.
Despite promising results, limitations include the study’s confinement to expert hepatopathologists, which may not generalize to general pathologists or community settings. Prospective validation in larger cohorts and real-world settings, including diverse pathologist expertise, will be essential for broad adoption. Furthermore, integration with other non-invasive fibrosis markers and clinical parameters could optimize diagnostic workflows.
Conclusion
AI-assisted digital pathology, specifically utilizing SHG/TPEF imaging and AI-derived quantitative fibrosis metrics, significantly enhances inter-pathologist agreement in fibrosis staging for MASH. This improvement is most pronounced in early fibrosis stages critical for clinical trial eligibility and disease monitoring. The enhanced concordance potentially reduces adjudication burden and increases clinical trial power, thereby offering meaningful advances in clinical research efficiency and treatment evaluation.
Future work should focus on validating AI tools in prospective, multi-center studies, expanding access beyond expert centers, and integrating such platforms into routine pathology practice to improve patient care and accelerate therapeutic discovery in MASH.
References
1. Abdurrachim D, Lek S, Ong CZL, et al. Utility of AI digital pathology as an aid for pathologists scoring fibrosis in MASH. J Hepatol. 2025 May;82(5):898-908. doi:10.1016/j.jhep.2024.11.032 IF: 33.0 Q1 .2. Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017;65(5):1557-1565.
3. Kalia HS, Gaglio PJ, Hooker CA, et al. Histological agreement of NASH fibrosis among pathologists in clinical practice. Mod Pathol. 2020;33(7):1418-1426.
4. Liu Y, Guo J, Chen Y, et al. Use of second harmonic generation microscopy for collagen assessment in liver fibrosis. Biosensors (Basel). 2021;11(5):162.