Highlight
1. Tirzepatide at a low dose of 5 mg weekly significantly increased insulin sensitivity measured by glucose infusion rate (GIR) within 12 weeks.
2. HbA1c and body weight notably decreased alongside reductions in fat mass and glucagon levels.
3. Improvement in insulin sensitivity occurred independent of weight loss, suggesting direct metabolic effects.
4. No significant correlations were found between GIR changes and typical metabolic markers, indicating a complex mechanism of action.
Study Background and Disease Burden
Type 2 diabetes mellitus (T2DM) and obesity constitute interrelated global health challenges, with insulin resistance as a central pathophysiological hallmark. Japan faces a significant and growing burden of T2DM complicated by obesity, particularly as metabolic derangements contribute to cardiovascular and microvascular complications, thereby increasing morbidity and mortality.
Current therapeutic approaches aiming to improve glycaemic control and reduce cardiovascular risk include agents that enhance insulin sensitivity. Tirzepatide, a novel dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist, has demonstrated promising efficacy in glycaemic control and weight reduction in large phase 3 trials. However, direct in vivo evidence of early insulin sensitization effects, particularly assessed with the gold-standard hyperinsulinaemic-euglycaemic clamp technique, remains limited, especially in the Japanese population.
Study Design
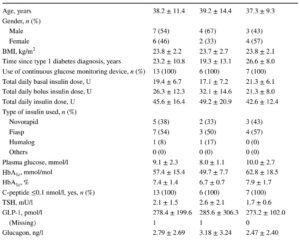
This was a prospective, single-arm, open-label, single-centre study involving 16 Japanese individuals with obesity and T2DM. Participants initially received tirzepatide 2.5 mg once weekly for 4 weeks, followed by dose escalation to 5 mg once weekly for an 8-week maintenance period.
The primary endpoint was the change in insulin sensitivity, quantitatively measured by the glucose infusion rate (GIR) during the hyperinsulinaemic-euglycaemic clamp test before and after treatment. Secondary endpoints included changes in HbA1c, body weight, body composition (muscle mass, fat mass, fat percentage), lipid profile, plasma glucagon level, and indices of insulin resistance and beta-cell function (HOMA2-IR and HOMA2-%β). Relationships between these variables and GIR changes were explored by simple linear regression analyses.
Key Findings
All 16 participants completed the 12-week study. After treatment, GIR increased significantly from 3.21 to 5.16 mg min-1 kg-1 (p<0.001), indicating a marked improvement in insulin sensitivity.
HbA1c decreased from 63.4 mmol/mol (7.95%) to 43.6 mmol/mol (6.14%) (p<0.001), demonstrating substantial improvement in glycaemic control. Body weight was reduced by an average of 4.9 kg (5.0%, p<0.001). Examining body composition revealed significant decreases in muscle mass (−1.8%), fat mass (−9.1%), and fat percentage (−4.5%), highlighting preferential fat loss.
Plasma glucagon levels decreased from 28.8 pg/ml to 20.8 pg/ml (significant reduction), consistent with an inhibitory effect on alpha-cell secretion. However, lipid profile changes were not prominently reported.
Notably, simple linear regression analyses did not identify any significant correlations between changes in GIR and other clinical variables such as weight loss, HbA1c reduction, or glucagon levels, suggesting that tirzepatide’s insulin-sensitizing effects may act via mechanisms independent from these measured parameters.
No serious adverse events or safety concerns were reported in this short-term study, though limited sample size and duration restrict safety conclusions.
Expert Commentary
This study provides compelling early evidence of tirzepatide’s capacity to improve insulin sensitivity independently of weight loss in Japanese patients with obesity and T2DM. The use of the hyperinsulinaemic-euglycaemic clamp, the gold standard for assessing insulin sensitivity, strengthens the validity of these findings.
The dissociation between improvements in GIR and conventional metabolic markers underscores the complex multifactorial mechanisms of tirzepatide, likely involving enhanced incretin signaling, modulation of glucagon secretion, and possible direct peripheral insulin-sensitizing effects.
Limitations include the small sample size and the single-arm design without a comparator, which restrict generalizability. The short duration also precludes assessment of long-term efficacy and safety. Furthermore, the study was confined to a Japanese cohort, and extrapolation to other ethnicities requires caution due to differences in diabetes pathophysiology.
Future studies with larger, randomized controlled designs and mechanistic investigations are warranted to elucidate tirzepatide’s full spectrum of metabolic actions and clinical benefits.
Conclusion
This prospective open-label study demonstrates that tirzepatide administered at a low dose (5 mg weekly) over 12 weeks significantly enhances insulin sensitivity in obese Japanese individuals with type 2 diabetes, as measured by the hyperinsulinaemic-euglycaemic clamp technique. Importantly, this early metabolic benefit appears partly independent of weight reduction.
The findings support tirzepatide’s therapeutic potential beyond glycaemic control and weight loss, highlighting its role as a promising agent for targeting insulin resistance. As insulin resistance is a fundamental driver of T2DM progression and complications, these results may have important implications for optimizing management strategies in this population.
Continued research should focus on confirming these effects in larger cohorts, establishing long-term safety, and clarifying the mechanistic pathways involved.
References
Yamaguchi Y, Kuwata H, Imura M, Moyama S, Usui R, Matsushiro M, Hamamoto Y, Yamada Y, Seino Y, Yamazaki Y. Early induction of insulin sensitisation treated by tirzepatide: a prospective, single-arm, open-label study in Japanese individuals with obesity and type 2 diabetes. Diabetologia. 2025 Oct;68(10):2151-2155. doi: 10.1007/s00125-025-06493-5. Epub 2025 Jul 22. PMID: 40694059.
Fang Z, Chen J, Yu Z, et al. Tirzepatide improves glycemic control and body weight in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2024 Mar;26(3):588-599. doi: 10.1111/dom.14623.
Madsbad S. Incretin therapies in type 2 diabetes: focus on insulin resistance and β-cell function. Diabetes Obes Metab. 2022 Jun;24 Suppl 2:3-12. doi: 10.1111/dom.14676.