Highlight
– KEYNOTE-585 trial adding pembrolizumab to fluorouracil and cisplatin chemotherapy improved pathologic complete response but failed to significantly improve event-free survival (EFS).
– MATTERHORN trial demonstrated that durvalumab combined with FLOT chemotherapy significantly improved EFS in resectable esophagogastric cancer.
– Use of the FLOT triplet chemotherapy backbone is critical for improved outcomes and effective integration of immunotherapy.
– Preoperative immunotherapy addition is essential; post-operative immunotherapy alone has not shown benefit in esophagogastric cancer.
Study Background and Disease Burden
Esophagogastric adenocarcinoma remains a challenging malignancy with significant morbidity and mortality worldwide. Surgical resection combined with systemic therapy has long been the cornerstone of curative intent treatment. Over the past two decades, perioperative chemotherapy has increasingly become standard of care, replacing surgery-first approaches, especially in Western countries. However, the optimal chemotherapy regimen and integration of immunotherapy remain under investigation. The role of radiotherapy added to perioperative chemotherapy has not demonstrated clear benefit. Immunotherapy targeting the PD-1/PD-L1 axis shows promise in advanced stages but has not been definitively established in the perioperative setting for resectable disease.
Study Design
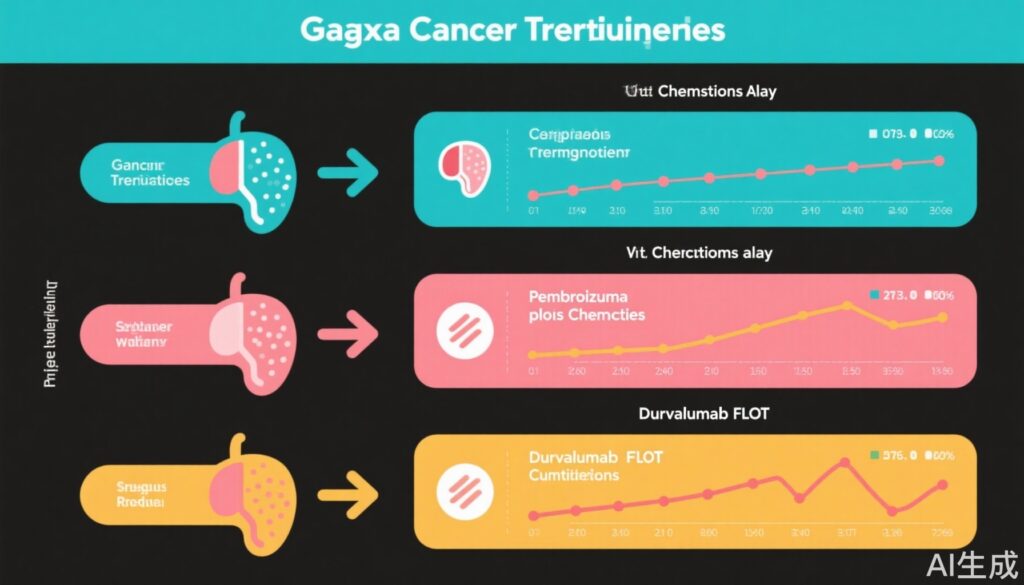
The KEYNOTE-585 trial was a global phase III randomized controlled trial evaluating whether the addition of pembrolizumab, a PD-1 inhibitor, to perioperative chemotherapy with fluorouracil (FU) and cisplatin improves outcomes in patients with resectable esophagogastric cancer. The primary endpoint was event-free survival (EFS), with a coprimary endpoint of pathologic complete response (pCR). Due to regional differences, particularly in Asia, the chemotherapy backbone was the FU-cisplatin doublet rather than FLOT (FU, leucovorin, oxaliplatin, and docetaxel). A late amendment added a smaller cohort randomized to FLOT plus pembrolizumab or placebo.
In contrast, the MATTERHORN trial randomized 948 patients with resectable esophagogastric cancer to perioperative FLOT chemotherapy with or without durvalumab, a PD-L1 inhibitor, with EFS as the primary endpoint and overall survival (OS) as a key secondary endpoint. All patients received the FLOT triplet chemotherapy consistent with contemporary standards.
Key Findings
KEYNOTE-585: The trial did not meet its primary endpoint of significantly improved EFS with the addition of pembrolizumab (HR 0.81; p>0.05), despite a nearly 20-month median numerical improvement from 25.7 to 44.4 months. The pCR rate was significantly higher with pembrolizumab. Overall survival exhibited numerical improvement (median OS 71.8 vs. 55.7 months), but statistical testing was limited due to the primary endpoint not being met. The late-added FLOT cohort did not change these outcomes significantly. Limitations include the use of FU and cisplatin doublet rather than FLOT, potentially attenuating efficacy and power.
MATTERHORN: Durvalumab added to FLOT chemotherapy significantly improved EFS (2-year EFS 67.5% vs. 58.5%; HR 0.71; statistically significant). OS trended favorably at 2 years (75.7% vs. 70.4%). A 12% improvement in pCR was observed with durvalumab. Importantly, the standardized use of the taxane-containing FLOT regimen as the chemotherapy backbone aligns with established standards and likely contributed to the positive outcomes.
Cross-Trial Comparisons: Although direct cross-trial comparisons are limited by differing designs and populations, efficacy outcomes for FLOT control arms have been consistent across trials. The similar survival and pCR rates in the immunotherapy plus FLOT arms of both trials support the biological activity of the immunotherapy agents when combined with robust chemotherapy backbones.
Safety: Both trials reported no increased surgical morbidity or unacceptable toxicities related to immunotherapy addition, supporting the feasibility of perioperative immunotherapy integration.
Expert Commentary
KEYNOTE-585’s failure to meet its primary endpoint highlights the critical importance of chemotherapy backbone selection. The inferior doublet regimen without a taxane may partly explain the lack of statistically significant survival benefit despite improved pathological responses. FLOT’s consistency across trials underscores the necessity of a triplet chemotherapy platform that maximizes tumor cytoreduction to facilitate immunotherapy synergy.
The difference between pembrolizumab (anti-PD-1) and durvalumab (anti-PD-L1) may also contribute but is less likely the primary factor given similar immune checkpoint inhibition pathways and comparable pCR enhancements seen in both trials.
Preoperative immunotherapy appears essential, as trials evaluating adjuvant immunotherapy alone post-surgery (e.g., ATTRACTION-5) have not demonstrated benefits, reinforcing a biological rationale for early immune priming before surgical tumor resection.
Regional treatment variations, particularly between Western and Asian gastric cancer management strategies, may affect trial outcomes and underscore the need for globally unified regimens like FLOT combined with immunotherapy.
Conclusion
The divergent outcomes of KEYNOTE-585 and MATTERHORN trials in perioperative immunotherapy for resectable esophagogastric cancer provide important lessons:
- Perioperative immunotherapy improves pathological response; however, survival benefits require an optimized chemotherapy platform, notably the FLOT regimen.
- Preoperative immunotherapy incorporation is critical; post-operative immunotherapy alone is insufficient.
- Global adoption of perioperative FLOT plus durvalumab establishes a new standard of care for operable esophagogastric adenocarcinoma.
- Future directions involve biomarker-driven patient selection, exploring novel immune enhancers, and integrating emerging targeted agents for improved outcomes.
These findings will inform clinical practice and ongoing trial designs, aiming to further improve long-term survival in this challenging cancer.
References
1. Ilson DH. KEYNOTE-585 Fails While Matterhorn Succeeds in Gastric Cancer: What Lessons Can We Learn? J Clin Oncol. 2025 Aug 19:JCO2501439.
2. Al-Batran SE et al. Perioperative chemotherapy with FLOT versus ECF/ECX in gastric or gastro-esophageal junction adenocarcinoma (FLOT4): Lancet. 2019;393(10184):1948-1957.
3. Shah MA et al. MATTERHORN Study: Durvalumab plus FLOT in Resectable Gastric Cancer, ASCO GI 2024 Abstract.
4. Ohtsu A et al. ATTRACTION-5: Nivolumab plus chemotherapy after upfront gastrectomy. JCO. 2023.
5. Wang Z et al. RESOLVE Trial favoring perioperative chemotherapy over adjuvant alone in gastric cancer. J Clin Oncol. 2023.
6. Kang YK et al. PRODIGY Trial: Preoperative chemotherapy in gastric cancer. J Clin Oncol. 2022.