Highlights
- The novel cAMP-biased GLP-1 receptor agonist ecnoglutide showed non-inferior efficacy to dulaglutide in HbA1c reduction at 32 and 52 weeks in type 2 diabetes patients receiving metformin monotherapy.
- Ecnoglutide 1.2 mg weekly provided statistically significant but clinically modest superior HbA1c reduction versus dulaglutide 1.5 mg.
- Both ecnoglutide doses (0.6 mg and 1.2 mg) demonstrated safety and tolerability profiles comparable to dulaglutide.
- These findings support ecnoglutide as a potential new treatment option, expanding GLP-1 RA therapeutic choices with a biased signaling mechanism.
Background
Type 2 diabetes mellitus (T2DM) imposes significant morbidity worldwide, primarily due to chronic hyperglycemia and associated cardiovascular risks. Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have transformed T2DM management by providing effective glycemic control, weight loss, and cardiovascular benefits. Dulaglutide, a well-established weekly GLP-1 RA, is a standard treatment. Yet, novel agents targeting biased signaling pathways, such as preferential cAMP activation over β-arrestin recruitment, may optimize therapeutic effects and safety.
Ecnoglutide is a recently developed cAMP-biased GLP-1 RA designed to preferentially activate intracellular cAMP pathways while minimizing β-arrestin recruitment, potentially enhancing efficacy or reducing adverse effects. This phase 3 trial (EECOH-2) assessed ecnoglutide’s efficacy and safety compared to dulaglutide in adults with T2DM inadequately controlled on metformin monotherapy.
Key Content
EECOH-2 Trial Design and Population
A 52-week, multicentre, open-label, randomized, non-inferiority phase 3 study was conducted at 52 hospitals in China. Eligible adults aged 18–75 years had T2DM, BMI 20–35 kg/m2, and elevated glucose despite stable metformin monotherapy. Participants (N=623 randomized; 621 in full analysis set) were assigned 1:1:1 to once-weekly subcutaneous injections of ecnoglutide 0.6 mg, ecnoglutide 1.2 mg, or dulaglutide 1.5 mg.
Primary and Secondary Endpoints
The primary efficacy endpoint was the mean change in glycated hemoglobin (HbA1c) from baseline to week 32, testing non-inferiority of ecnoglutide doses to dulaglutide with a 0.4% margin, and superiority testing for ecnoglutide 1.2 mg. Secondary endpoints included HbA1c changes at week 52 and safety assessments including adverse event incidence and treatment discontinuations.
Efficacy Outcomes
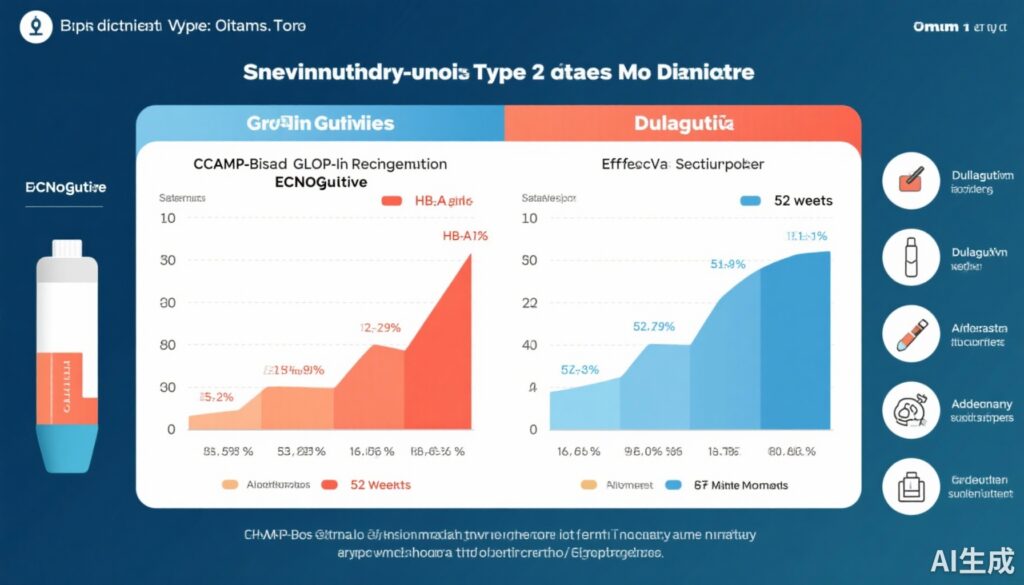
At week 32, mean HbA1c reductions were 1.91% (SE 0.05) for ecnoglutide 0.6 mg, 1.89% (SE 0.05) for ecnoglutide 1.2 mg, and 1.65% (SE 0.05) for dulaglutide 1.5 mg. Treatment differences versus dulaglutide were -0.26% (95% CI -0.39 to -0.13) for ecnoglutide 0.6 mg and -0.24% (95% CI -0.38 to -0.11; p=0.0002 for superiority) for ecnoglutide 1.2 mg, indicating non-inferiority for both doses and statistical superiority for the higher dose. However, the magnitude was not deemed clinically relevant. HbA1c reductions persisted through week 52.
Safety and Tolerability
Adverse event-related treatment discontinuations were low and comparable across groups: 3% for ecnoglutide 0.6 mg, 4% for ecnoglutide 1.2 mg, and 3% for dulaglutide. Gastrointestinal symptoms were the most commonly reported events, consistent with the GLP-1 RA class profile. No new safety signals or severe adverse events were identified.
Comparative and Contextual Evidence
Other GLP-1 RAs such as dulaglutide, semaglutide, and liraglutide have demonstrated cardiovascular benefit, weight loss, and glycemic efficacy. Novel agents like tirzepatide, a dual GIP/GLP-1 RA, have shown superior HbA1c and weight reduction compared with dulaglutide but may confer limited incremental cardiovascular benefit beyond GLP-1 RA therapy alone. Ecnoglutide’s biased agonism represents a new mechanistic approach within the GLP-1 RA class.
Network meta-analyses indicate that tirzepatide outperforms dulaglutide in HbA1c and weight reduction but with similar safety. Real-world prescribing patterns and economic evaluations favor dulaglutide or oral semaglutide over some competitors based on cost-effectiveness profiles.
Further, GLP-1 RAs have demonstrated benefits beyond glycemic control, including lipid profile improvements and no increased risk of sight-threatening diabetic retinopathy relative to other agents.
Expert Commentary
The EECOH-2 phase 3 trial provides robust evidence that ecnoglutide, with its novel cAMP-biased mechanism, is an effective and safe alternative to dulaglutide for glycemic control in T2DM patients on metformin. The statistically significant superiority of ecnoglutide 1.2 mg in HbA1c reduction, though modest, suggests potential incremental benefit but may not warrant a change in clinical practice alone.
The open-label design and geographic restriction to Chinese centers may limit global generalizability, though patient demographics are broadly representative. The tolerability profile aligns with expectations for GLP-1 RAs, with gastrointestinal side effects being predominant.
Mechanistically, biased GLP-1 receptor agonism may optimize downstream signaling to achieve clinical effects with potentially differing safety or efficacy profiles, meriting exploration in further clinical trials and real-world studies. The competitive landscape includes dual agonists and oral formulations, emphasizing the need for personalized therapy considering efficacy, safety, and patient preference.
Conclusion
Ecnoglutide represents a promising addition to the GLP-1 RA class, providing effective HbA1c reductions sustained to 52 weeks with comparable safety to dulaglutide. While its cAMP-biased receptor activation offers a novel pharmacological approach, clinical benefits over established agents appear modest. Future research should evaluate long-term cardiovascular outcomes, cost-effectiveness, and broader population applicability. These findings enrich therapeutic options for individualized care in type 2 diabetes.
References
- He Y, Mi N, Cheng Z, Xue H, Han J, Wang H, et al. Efficacy and safety of cAMP-biased GLP-1 receptor agonist ecnoglutide versus dulaglutide in patients with type 2 diabetes and elevated glucose concentrations on metformin monotherapy (EECOH-2): a 52-week, multicentre, open-label, non-inferiority, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2025 Oct;13(10):863-873. doi: 10.1016/S2213-8587(25)00196-2. PMID: 40854315.
- Ito S, et al. Network Meta-analysis of Tirzepatide versus GLP-1 Receptor Agonists in T2DM with Basal Insulin. Diabetes Ther. 2025 Jun;16(6):1279-1311. PMID: 40214900.
- Chow F, et al. Can Dual Incretin Receptor Agonists Exert Better Cardiovascular Protection than Selective GLP-1 Receptor Agonists? Highlights from SURPASS-CVOT. Diabetes Ther. 2025 Aug;13(10):863-873. PMID: 40886230.
- Hernandez A, et al. Economic Evaluation of Liraglutide vs Dulaglutide or Oral Semaglutide in T2DM: Systematic Review. Diabetol Metab Syndr. 2025 Aug 27;17(1):358. PMID: 40859274.
- Singh D, et al. Risk of Sight-Threatening Diabetic Retinopathy With GLP-1 RA Use in Routine Clinical Practice. Ophthalmol Retina. 2025 Aug 5:S2468-6530(25)00355-0. PMID: 40774571.