Introduction
Refractory chronic cough (RCC) represents a significant clinical challenge, characterized by persistent cough lasting over eight weeks despite investigation and treatment for known causes or lacking identifiable etiology. Affecting a notable proportion of adults, RCC often impairs patients’ physical, psychological, and social well-being. Currently, there is no widely accessible, approved therapy for RCC, and available treatments like neuromodulators are limited by efficacy concerns and adverse effects. Recent advances focus on peripheral targets such as the P2X3 receptor, an ATP-gated ion channel implicated in sensory neuron activation and cough reflex hypersensitivity. Camlipixant is a selective P2X3 antagonist evaluated for efficacy and safety in RCC management. This article critically reviews the SOOTHE phase 2b randomized trial that investigated camlipixant in adults with RCC and elevated baseline cough frequency.
Study Design and Methods
SOOTHE was a multicenter, double-blind, placebo-controlled, phase 2b dose-finding trial enrolling adults aged 18–80 years with RCC duration ≥1 year and awake cough frequency ≥25 coughs/h. A 16-day single-blind placebo run-in aimed to stabilize cough frequency and mitigate placebo effects. Post run-in, 310 patients were randomized (1:1:1:1) to receive camlipixant at 12.5 mg, 50 mg, or 200 mg twice daily or placebo for 4 weeks. Patients were stratified at randomization by awake cough frequency (<45 or ≥45 coughs/h) to balance baseline severity across arms.
The primary endpoint was the change from baseline to Day 28 in objective 24-hour cough frequency measured using the VitaloJAK automated cough monitor, analyzed on the natural log scale. Secondary endpoints included changes at Day 15, awake and nighttime cough frequency, responder analyses at different cough reduction thresholds (≥30%, ≥50%, ≥70%), patient-reported outcomes including cough severity (CS-VAS) and cough-related quality of life (Leicester Cough Questionnaire; LCQ), and safety assessments.
An exploratory cohort with baseline awake cough frequency 10 to <25 coughs/h was included and randomized to camlipixant 200 mg or placebo, though not powered for efficacy comparisons.
Key Findings
Of 310 randomized patients, 249 comprised the main population (awake cough frequency ≥25 coughs/h). Baseline characteristics were balanced, with the majority female (~78%) and mean cough duration exceeding 10 years.
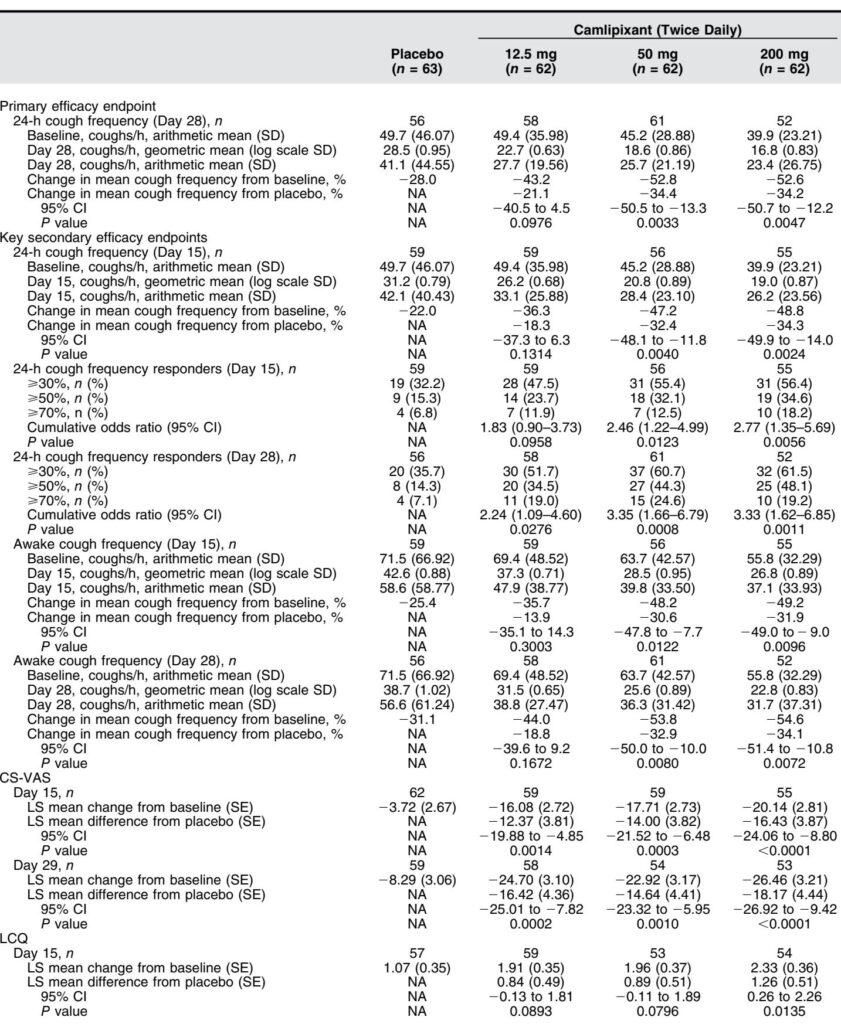
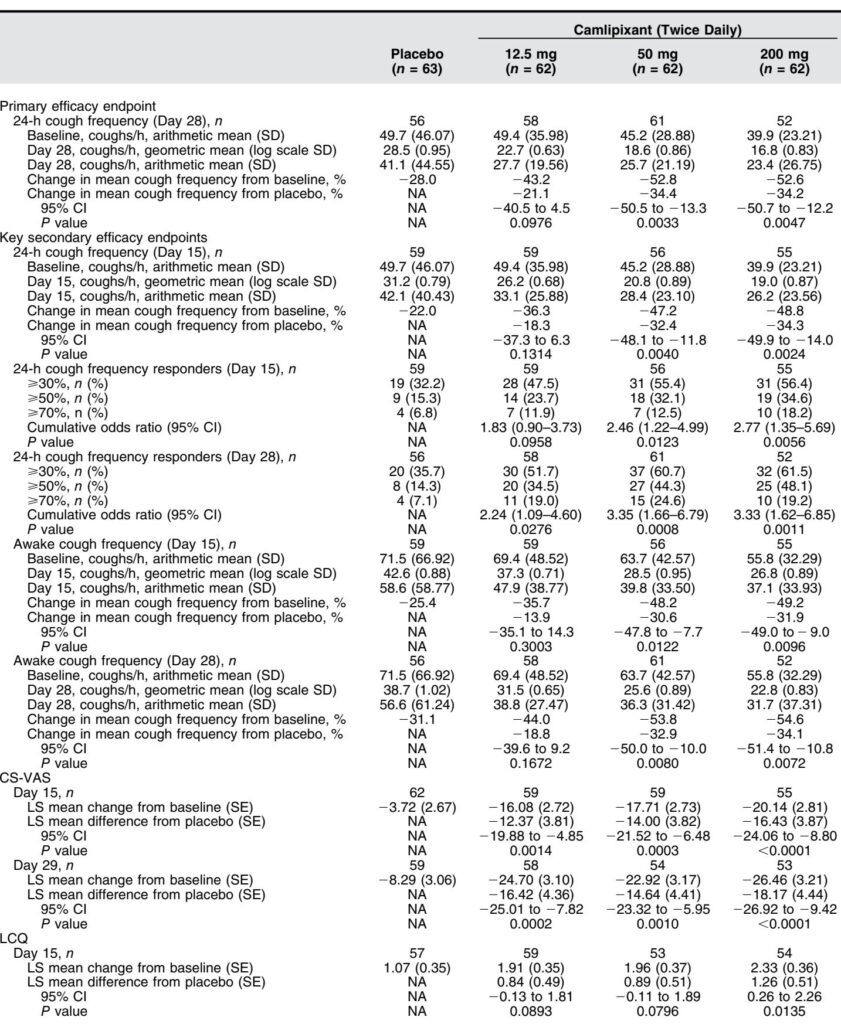
Primary Endpoint: Camlipixant at 50 mg and 200 mg twice daily significantly reduced 24-hour cough frequency compared with placebo at Day 28 by 34.4% (95% CI, -50.5 to -13.3; P=0.0033) and 34.2% (95% CI, -50.7 to -12.2; P=0.0047), respectively. The 12.5 mg dose showed a non-significant 21.1% reduction.
Secondary Endpoints:
– Significant reductions in 24-hour cough frequency were observed as early as Day 15 in the 50 mg and 200 mg arms.
– Awake cough frequency decreased significantly in these dose groups at Days 15 and 28.
– Responder analyses revealed higher proportions of patients achieving clinically meaningful cough reductions (≥30%, ≥50%, ≥70%) with camlipixant versus placebo.
– Nighttime cough frequency reductions trended favorably but did not reach statistical significance.
– Patient-reported outcomes showed consistent improvements; CS-VAS scores decreased notably with camlipixant doses at Days 15 and 29, and LCQ scores improved, reflecting enhanced quality of life, particularly at the 200 mg dose.
Safety and Tolerability: Treatment-emergent adverse events (AEs) were similar between camlipixant and placebo arms. The most common AEs included nausea and taste alterations (dysgeusia), reported in ≤6.5% of camlipixant-treated patients and none in placebo. All taste-related AEs were mild to moderate, and no patients discontinued treatment due to taste issues. No serious treatment-emergent AEs were reported.
Expert Commentary
The SOOTHE trial robustly demonstrates that camlipixant reduces cough frequency and improves patient-reported outcomes in a well-characterized RCC population with elevated baseline cough frequency. The inclusion of a placebo run-in period and baseline stratification based on awake cough frequency enhanced patient selection and outcome reliability.
These findings align with prior studies of P2X3 antagonists, such as gefapixant and eliapixant, demonstrating similar efficacy magnitudes but with potentially improved tolerability profiles, especially regarding taste disturbances. The lower incidence of taste adverse effects with camlipixant may reflect its selective P2X3 antagonism, minimizing P2X2/3 receptor blockade associated with altered taste perception.
Interestingly, efficacy appears linked to baseline cough frequency, supporting stratification approaches focusing on patients with more severe cough. This phenomenon warrants further mechanistic investigation. The trial’s relatively short duration precludes assessment of long-term efficacy and safety, an important consideration given RCC’s chronic nature.
Conclusions
Camlipixant, administered twice daily at 50 mg and 200 mg, significantly reduces objective cough frequency and improves life quality metrics in patients with refractory chronic cough and high baseline cough frequency. Its favorable safety and tolerability profile, especially regarding taste alterations, supports its potential as a novel targeted therapy for RCC.
Ongoing phase 3 trials (CALM-1 and CALM-2) will further elucidate camlipixant’s therapeutic profile and establish its clinical utility in this underserved patient population.
References
Smith JA, Birring SS, Blaiss MS, McGarvey L, Morice AH, Sher M, Carroll KJ, Garin M, Lanouette S, Shaw J, Yang R, Bonuccelli CM. Camlipixant in Refractory Chronic Cough: A Phase 2b, Randomized, Placebo-controlled Trial (SOOTHE). Am J Respir Crit Care Med. 2025 Jun;211(6):1038-1048. doi: 10.1164/rccm.202409-1752OC. PMID: 40043302; PMCID: PMC12180140.