Highlights
- Prademagene zamikeracel, an autologous gene-modified cellular therapy, achieved ≥50% healing in 81% of treated chronic wounds at 24 weeks, compared to 16% with standard care.
- Significant pain reduction was observed in treated wounds, with a mean decrease of 3.07 points versus 0.90 in controls.
- No serious treatment-related adverse events were reported, supporting a favorable safety profile.
- This study represents the first phase 3 trial of a gene therapy directly targeting the underlying genetic defect in RDEB wounds.
Clinical Background and Disease Burden
Recessive dystrophic epidermolysis bullosa (RDEB) is a rare, inherited, and devastating skin disorder caused by biallelic mutations in the COL7A1 gene, which encodes type VII collagen. This protein is crucial for anchoring the epidermis to the underlying dermis. Its deficiency results in extreme skin fragility, leading to chronic blistering, erosions, and large non-healing wounds. The disease is associated with severe pain, recurrent infections, scarring, contractures, and a markedly increased risk of aggressive cutaneous squamous cell carcinoma. The burden on patients and caregivers is profound, spanning physical, psychological, and socioeconomic domains. Current standards of care are palliative, focusing on wound care, infection prevention, and pain management, but do not address the underlying molecular defect. There is an urgent unmet need for disease-modifying therapies that can promote sustained wound healing and improve quality of life.
Research Methodology
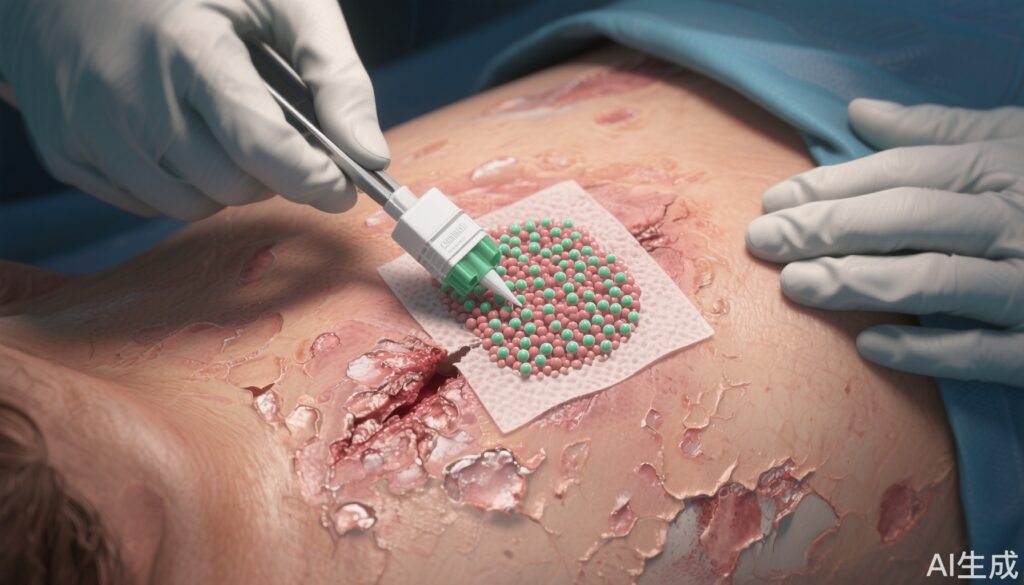
The VIITAL study was a randomized, open-label, intrapatient-controlled, phase 3 trial conducted at two US centers. Eligible participants were 6 years or older with a confirmed clinical and genetic diagnosis of RDEB, required to have at least two chronic wounds larger than 20 cm², no immune response to type VII collagen, and expression of the NC1 fragment of the protein. For each patient, large, chronic wounds were matched into pairs by size, chronicity, and location, then randomly assigned (1:1) to receive either prademagene zamikeracel or standard of care. Prademagene zamikeracel is an autologous, COL7A1 gene-modified keratinocyte sheet surgically sutured onto the wound bed. Up to six wounds could be treated per patient. The two coprimary endpoints were the proportion of wounds achieving at least 50% healing and reduction in wound-associated pain at 24 weeks. Safety was evaluated in all patients and wounds, including non-randomized ones.
Key Findings
Out of 15 screened patients, 11 were enrolled, contributing 86 wounds (43 pairs). The median participant age was 21 years (IQR 17-30), with a 64% female preponderance. At week 24, 35 of 43 (81%) wounds treated with prademagene zamikeracel achieved at least 50% healing, compared with only 7 of 43 (16%) control wounds—a mean difference of 67% (95% CI 50 to 89; p<0.0001), demonstrating robust statistical significance. Pain outcomes also favored the gene therapy group: mean pain score change from baseline was -3.07 for treated versus -0.90 for controls (mean pairwise difference -2.23; 95% CI -3.45 to -0.66; p=0.0002). Importantly, no serious treatment-related adverse events were observed, and overall tolerability was excellent.
| Endpoint | Prademagene Zamikeracel | Control | p-value |
|---|---|---|---|
| ≥50% wound healing at 24 weeks | 81% (35/43 wounds) | 16% (7/43 wounds) | <0.0001 |
| Mean pain reduction (0-10 scale) | -3.07 | -0.90 | 0.0002 |
| Serious treatment-related AEs | None | None | N/A |
Mechanistic Insights and Biological Plausibility
Prademagene zamikeracel leverages ex vivo gene modification of autologous keratinocytes to restore expression of functional type VII collagen. Once applied to the wound, these genetically corrected sheets establish new epidermal layers capable of producing anchoring fibrils, thereby restoring dermal-epidermal cohesion and promoting durable wound closure. This targeted approach directly addresses the molecular pathology of RDEB, setting it apart from prior therapies that focus solely on symptom management. Preclinical and early-phase clinical studies have shown that such gene-corrected sheets can survive, engraft, and synthesize functional protein in vivo, lending strong biologic plausibility to the observed therapeutic effects.
Expert Commentary
The VIITAL trial represents a pivotal advancement in the treatment of RDEB, a disease for which meaningful progress has been elusive. The magnitude of wound healing observed with prademagene zamikeracel is unprecedented in this population and is complemented by meaningful reductions in pain—a critical outcome for patient quality of life. Notably, the absence of serious safety concerns addresses a major barrier to broader gene or cell-based therapies. While the study population was small, as is typical for ultra-rare diseases, the intrapatient-controlled design strengthens the reliability of the results.
Controversies and Limitations
The primary limitation of the VIITAL study is its small sample size (11 participants), which reflects both the rarity of RDEB and the stringent eligibility criteria. The open-label, non-blinded design introduces the potential for assessment bias, particularly for subjective endpoints such as pain. Furthermore, only patients expressing the NC1 fragment of type VII collagen and lacking pre-existing immune responses were included, which may limit generalizability to all individuals with RDEB. Long-term durability of wound healing, the risk of delayed adverse events, and the impact of repeated applications remain to be established through ongoing follow-up and additional studies.
Conclusion
Prademagene zamikeracel offers a transformative, gene-based therapeutic option for patients with large, chronic wounds due to RDEB. The VIITAL phase 3 trial demonstrates substantial improvements in wound healing and pain with an excellent safety profile. These results herald a new era in the management of this devastating disease, though longer-term outcomes and applicability to broader patient groups warrant further investigation. The study underscores the promise of precision cell and gene therapies for rare genetic skin disorders.
References
1. Eichstadt S, Tang JY, Solis DC, et al. Phase 1/2 study of gene-corrected autologous epidermal grafts in patients with recessive dystrophic epidermolysis bullosa. JAMA. 2019;322(15):1467-1475. doi:10.1001/jama.2019.13179
2. Fine JD, Bruckner-Tuderman L, Eady RAJ, et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70(6):1103-1126. doi:10.1016/j.jaad.2014.01.903
3. ClinicalTrials.gov. Prademagene zamikeracel in RDEB wounds (VIITAL). NCT04227106
4. Uitto J, et al. Progress in developing gene therapies for recessive dystrophic epidermolysis bullosa. Br J Dermatol. 2020;183(4):629-638. doi:10.1111/bjd.19183