Highlights
- Navigational bronchoscopy demonstrated noninferior diagnostic accuracy compared to transthoracic needle biopsy for peripheral pulmonary nodules (79.0% vs. 73.6%, respectively).
- The risk of pneumothorax was markedly lower with navigational bronchoscopy (3.3%) than with transthoracic needle biopsy (28.3%).
- Hospital admissions and chest tube placements for pneumothorax were significantly less frequent following navigational bronchoscopy.
- This multicenter randomized trial provides robust evidence to guide biopsy modality selection in patients with 10–30 mm peripheral pulmonary nodules.
Clinical Background and Disease Burden
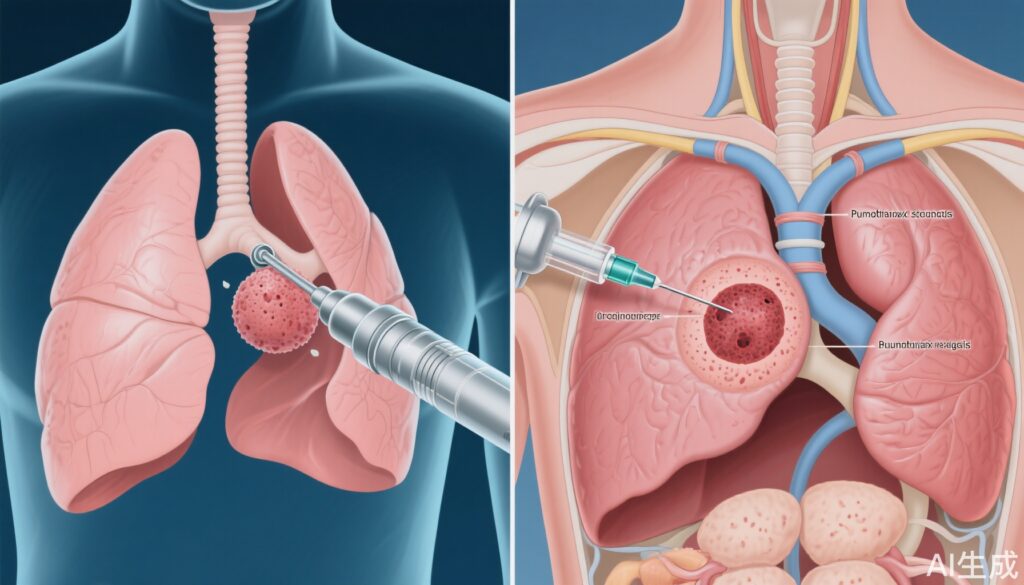
Pulmonary nodules are commonly detected incidentally or via lung cancer screening programs, with millions identified annually. The clinical imperative is to differentiate malignant from benign lesions accurately and safely, as management strategies and prognoses differ markedly. Peripheral pulmonary nodules (10–30 mm) pose a diagnostic challenge due to their location and the limitations of noninvasive imaging. Two principal biopsy modalities are used: computed tomography (CT)-guided transthoracic needle biopsy (TTNB) and advanced bronchoscopic techniques, notably navigational bronchoscopy (NB). TTNB is renowned for its diagnostic yield but carries a well-documented risk of complications, especially pneumothorax. Navigational bronchoscopy, utilizing electromagnetic or robotic guidance, offers a minimally invasive alternative, but its real-world diagnostic performance relative to TTNB has been unclear, especially for peripheral lesions.
Research Methodology
The VERITAS trial (NCT04250194), a multicenter, randomized, parallel-group, noninferiority study, sought to compare NB and TTNB in patients with intermediate- or high-risk peripheral pulmonary nodules (10–30 mm diameter). Conducted at seven centers across the United States, the trial enrolled 234 patients, randomly assigning them to undergo either NB or TTNB. The primary endpoint was diagnostic accuracy, defined as the proportion of patients with a biopsy diagnosis (malignant or specific benign) confirmed by clinical follow-up at 12 months, with a prespecified noninferiority margin of 10 percentage points. Secondary endpoints included procedural complication rates, notably the occurrence and severity of pneumothorax.
Key Findings
Of the 234 patients analyzed (with minimal loss to follow-up), specific diagnostic accuracy was achieved in 94/119 (79.0%) in the NB group and 81/110 (73.6%) in the TTNB group. The absolute difference was 5.4 percentage points (95% CI, –6.5 to 17.2), surpassing the noninferiority threshold (P=0.003 for noninferiority). Superiority was not statistically significant (P=0.17).
Complication rates differed sharply between groups. Pneumothorax occurred in only 3.3% (4/121) of NB patients, compared to 28.3% (32/113) in the TTNB arm. Clinically significant pneumothorax necessitating chest tube placement and/or hospital admission was also lower in the NB group (0.8% vs 11.5%).
These results provide high-level evidence that NB is not only as accurate as TTNB for peripheral nodules but is associated with a substantially safer procedural profile.
Mechanistic Insights and Biological Plausibility
The rationale for reduced pneumothorax risk with NB is clear: the bronchoscopic approach avoids traversing the pleura, in contrast to TTNB, which requires a transpleural needle path, inherently raising the risk of air leak. The diagnostic accuracy parity likely reflects advances in bronchoscopic navigation technology, improved sampling tools, and operator expertise, which together have closed the historical yield gap for peripheral nodules.
Expert Commentary
This trial addresses a persistent clinical dilemma: which biopsy modality to choose for peripheral pulmonary nodules when both are available. Recent expert consensus and guidelines (e.g., American College of Chest Physicians) have highlighted the need for individualized, risk-based selection, weighing diagnostic yield against procedural risk. The VERITAS trial’s robust, multicenter design adds critical clarity, suggesting NB can be preferentially considered—particularly in patients at high risk of pneumothorax or with comorbidities that would complicate recovery from TTNB complications.
Controversies or Limitations
Several limitations should be acknowledged. First, the study population was limited to nodules 10–30 mm; thus, findings may not generalize to smaller or larger lesions. Operator expertise and institutional resources (e.g., access to state-of-the-art bronchoscopic navigation systems) may vary, affecting reproducibility outside academic centers. The trial’s noninferiority margin, while clinically justified, is a matter of debate—some may argue for more stringent thresholds. The study was funded in part by Medtronic, a device manufacturer, though rigorous oversight and multicenter participation reduce bias concerns. Finally, secondary endpoints such as cost-effectiveness and patient-reported outcomes were not addressed and warrant future investigation.
Conclusion
The VERITAS trial delivers compelling evidence that navigational bronchoscopy matches TTNB in diagnostic accuracy for 10–30 mm peripheral pulmonary nodules while dramatically reducing the risk of pneumothorax and related complications. These findings support broader adoption of NB as a first-line diagnostic approach, especially in patients at elevated risk for TTNB-associated morbidity. Ongoing research should refine patient selection criteria, assess cost implications, and integrate patient-centered perspectives into procedural decision-making.
References
1. Lentz RJ, Frederick-Dyer K, Planz VB, et al. Navigational Bronchoscopy or Transthoracic Needle Biopsy for Lung Nodules. N Engl J Med. 2025 Jun 5;392(21):2100-2112. doi: 10.1056/NEJMoa2414059.
2. Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e142S-e165S. doi:10.1378/chest.12-2353.
3. Gould MK, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? ACCP evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e93S-e120S. doi:10.1378/chest.12-2351.