Highlights
- Mitochondrial donation via pronuclear transfer (PNT) is compatible with embryo viability and significantly reduces mtDNA disease transmission.
- Preimplantation genetic testing (PGT) effectively identifies embryos with low or absent pathogenic mtDNA heteroplasmy, providing an alternative for women with heteroplasmic variants.
- In a recent cohort, PNT led to 8 live births with markedly reduced pathogenic mtDNA load; PGT resulted in 18 live births with minimal heteroplasmy.
- An integrated approach using both PNT and PGT addresses the needs of women with homoplasmic and heteroplasmic mtDNA mutations, expanding reproductive options.
Clinical Background and Disease Burden
Mitochondrial DNA (mtDNA) diseases are severe, multisystem disorders resulting from pathogenic variants in mtDNA. Inherited exclusively through the maternal line, these diseases manifest with a spectrum of phenotypes affecting neurological, muscular, cardiac, and metabolic systems. Heteroplasmy—the co-existence of mutant and wild-type mtDNA within cells—determines disease severity and penetrance. Women harboring homoplasmic (all mtDNA copies affected) or high heteroplasmic pathogenic variants face a high risk of transmitting these diseases to their offspring. Current management options have been limited, often forcing difficult reproductive decisions.
Research Methodology
This study, published in the New England Journal of Medicine (Hyslop LA et al., 2025), evaluated two advanced reproductive interventions for women with pathogenic mtDNA variants: mitochondrial donation by pronuclear transfer (PNT) and preimplantation genetic testing (PGT).
Women with heteroplasmic mutations were offered PGT, allowing selection of embryos with low or undetectable mutant mtDNA. Those with homoplasmic or high-level heteroplasmic mutations, for whom PGT is not viable, were offered PNT. PNT involves transferring the nuclear genome from a fertilized egg of an affected woman into an enucleated fertilized donor egg with healthy mitochondria, followed by intracytoplasmic sperm injection (ICSI). Key endpoints included embryo viability, pregnancy rates, live births, and heteroplasmy levels in offspring.
Key Findings
In the cohort, 22 women underwent PNT and 39 underwent PGT, each following ICSI. Clinical pregnancies occurred in 36% (8/22) in the PNT group and 41% (16/39) in the PGT group. PNT led to 8 live births and 1 ongoing pregnancy, while PGT yielded 18 live births.
Of infants born after PNT, blood heteroplasmy levels ranged from undetectable to 16%. Notably, 6 infants had pathogenic mtDNA variant levels reduced by 95–100% compared to enucleated zygotes, and the remaining 2 showed 77–88% reduction. For PGT, 10 of 18 infants had heteroplasmy data, with levels from undetectable to 7%. These results indicate a marked reduction in the risk for mtDNA disease in offspring.
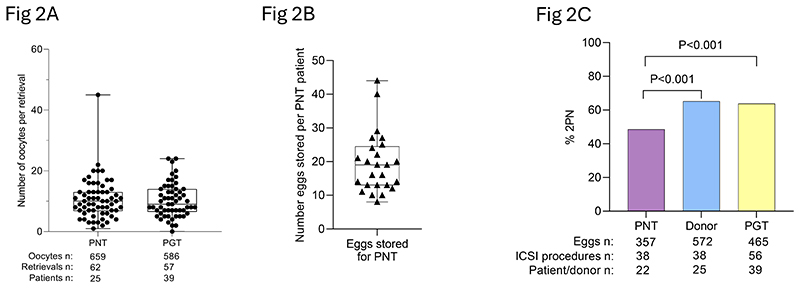
Oocyte retrieval and fertilization outcomes of pronuclear transfer patient, PGT patient and donor eggs.
Embryo viability after PNT was comparable to standard ART procedures. Oocyte retrieval and fertilization rates for PNT and PGT patients were generally lower than for healthy donor eggs, likely reflecting the underlying infertility issues in affected women. However, the difference in the proportion of normally fertilized eggs between patients and donors was significant (P<0.001), highlighting the technical challenges but also the feasibility of these interventions.
Mechanistic Insights
PNT fundamentally addresses the maternal inheritance of mtDNA by replacing cytoplasmic mitochondria while preserving the nuclear genetic identity of the parents. This approach circumvents the limitations of PGT when all maternal mtDNA is mutated (homoplasmy) or when high heteroplasmy precludes selection of disease-free embryos. The near-complete replacement of mutant mitochondria in offspring demonstrates the biological plausibility and efficiency of this method.
Expert Commentary
Dr. Doug Turnbull, a pioneer in mitochondrial research, notes, “These results represent a turning point for families affected by mitochondrial diseases. For the first time, women with homoplasmic mutations have a realistic option to have genetically related children with vastly reduced disease risk.”
The UK Human Fertilisation and Embryology Authority has recognized mitochondrial donation as a regulated clinical service, reflecting growing confidence in its safety and efficacy. However, long-term follow-up of children born through these techniques remains essential to fully establish their benefits and risks.
Controversies and Limitations
While the results are promising, several limitations warrant discussion:
- The cohort size remains modest, and long-term outcomes—particularly regarding health, development, and fertility of offspring—are not yet available.
- Technical complexity and resource requirements limit widespread accessibility.
- Residual low-level maternal mtDNA heteroplasmy persists in some cases after PNT, raising questions about potential late-onset or milder disease phenotypes.
- Ethical considerations, including germline modification and donor anonymity, continue to prompt debate in both medical and public domains.
Conclusion
Mitochondrial donation by pronuclear transfer and preimplantation genetic testing represent significant advances in reproductive medicine for women at risk of transmitting mtDNA disease. An integrated, individualized approach allows for tailored risk reduction based on heteroplasmy status, offering hope to families affected by these devastating conditions. Continued monitoring, further refinement of protocols, and robust ethical oversight will be essential as these technologies move toward broader clinical adoption.
References
1. Hyslop LA, Blakely EL, Aushev M, et al. Mitochondrial Donation and Preimplantation Genetic Testing for mtDNA Disease. N Engl J Med. 2025 Jul 31;393(5):438-449. doi: 10.1056/NEJMoa2415539 . Epub 2025 Jul 16. PMID: 40673696 ; PMCID: PMC7617940 .2. Gorman GS, Chinnery PF, DiMauro S, et al. Mitochondrial diseases. Nat Rev Dis Primers. 2016;2:16080. doi:10.1038/nrdp.2016.80 .3. Craven L, Tuppen HA, Greggains GD, et al. Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease. Nature. 2010;465(7294):82-85. doi:10.1038/nature08958 .