Background
Hand-foot syndrome (HFS) is a common, dose-limiting adverse effect of capecitabine, an oral chemotherapeutic frequently used in adjuvant treatment of human epidermal growth factor receptor 2 (HER2) negative early breast cancer. Grade 2 or 3 HFS causes erythema, edema, neuropathic pain, dysesthesia, blistering, and ulceration of palms and soles, substantially impairing patients’ quality of life and treatment adherence. Despite its clinical significance, current preventive strategies, including pyridoxine, celecoxib, and urea-based creams, have limitations due to efficacy or safety concerns. There is an unmet need for effective, safe approaches to reduce the incidence and severity of capecitabine-induced HFS.
Methylcobalamin, the active form of vitamin B12, has neuroprotective properties, promoting neuronal survival and regeneration by stimulating Erk1/2 and Akt pathways. Its efficacy in alleviating neuropathic pain and dysesthesia has been demonstrated in clinical settings. Given the suggested role of small fibre neuropathy in HFS pathogenesis, methylcobalamin was hypothesised to prevent or mitigate capecitabine-induced HFS in affected patients.
Study Design
This multicentre, double-blind, randomised, placebo-controlled phase 3 trial (ClinicalTrials.gov NCT05165069) was conducted in seven hospitals across China between January 2022 and February 2024. Eligible participants were women aged 18–75 years with centrally confirmed HER2 negative early breast cancer who required adjuvant capecitabine following either neoadjuvant chemotherapy without pathological complete response or lymph node metastasis after upfront surgery. They had Eastern Cooperative Oncology Group performance status 0 or 1 and adequate organ function. Key exclusions included conditions confounding HFS diagnosis and inability to swallow the investigational product.
Participants (n=234) were randomised 1:1 to receive oral methylcobalamin 0.5 mg thrice daily or placebo for up to 24 weeks concurrently with capecitabine dosed at 2000 mg/m2/day in two divided doses for eight 21-day cycles (14 days on, 7 days off). Blinding was maintained for participants, clinicians, and assessors.
Primary endpoint was the incidence of first occurrence of grade ≥2 HFS (per NCI-CTCAE v5.0) during treatment, adjudicated by two independent dermatologists with a third consulted for discrepancies. Secondary endpoints included rates of capecitabine dose reductions or discontinuations due to HFS, disease-free survival, overall survival, quality of life assessed by HF-QoL, QLQ-C30, and QLQ-BR23 questionnaires, and adverse event profiles.
Key Findings
Among 234 randomised patients (median age 50 years; 53% premenopausal; 67% triple negative; 94% prior chemotherapy), 117 received methylcobalamin and 117 placebo. Baseline demographic and clinical variables were well balanced. Compliance exceeded 98% for both groups.
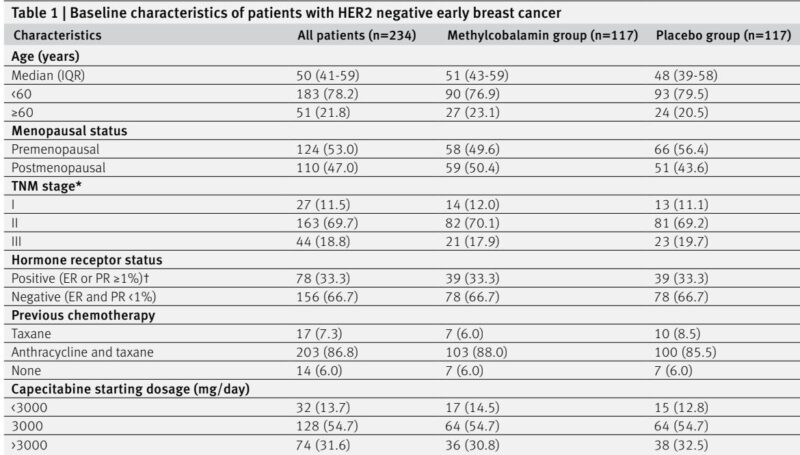
The primary endpoint incidence of grade ≥2 HFS was significantly lower in the methylcobalamin group: 14.5% (17/117) versus 29.1% (34/117) in placebo (risk difference -14.5%; 95% CI -24.9 to -4.1; one-sided P=0.003). Adjusted analyses controlling for hormone receptor status, study centre, and patient characteristics confirmed the robustness of this reduction (adjusted risk difference -15.7%, P=0.007). Subgroup analyses showed consistent benefits across age, menopausal status, TNM stage, hormone receptor status, prior chemotherapy, and capecitabine dose.
Secondary efficacy outcomes included fewer patients in the methylcobalamin group requiring capecitabine dose reductions (6.8% vs 11.1%) or discontinuations due to HFS (0.9% vs 2.6%), although differences were not statistically significant. Disease-free and overall survival rates were similar between groups at median 24-month follow-up.
Quality of life metrics showed a significantly smaller increase in hand-foot syndrome related daily activity interference scores at cycle 4 in the methylcobalamin group (median increase 6.0 vs 8.0, P=0.005); however, differences leveled off by treatment completion. Other global and breast cancer-specific quality of life measures were comparable.
Adverse event profiles were similar between groups (75.2% vs 81.2% with any-grade toxicity), with no methylcobalamin-specific adverse events or serious adverse events reported. Hematologic toxicities were the most common, consistent with known capecitabine effects. No patients discontinued capecitabine due to non-HFS toxicity.
Expert Commentary
This rigorous phase 3 trial demonstrates that oral methylcobalamin significantly reduces the incidence of clinically meaningful grade ≥2 capecitabine-induced hand-foot syndrome without increasing toxicity, representing an important advance in supportive care for this patient population. Its neuroprotective and nerve regenerative properties likely address underlying small fibre neuropathy implicated in HFS pathogenesis. The safety, oral administration, and low cost of methylcobalamin enhance its clinical utility.
Compared to previous agents such as celecoxib or topical diclofenac, methylcobalamin avoids cardiovascular risks and systemic absorption concerns, respectively. Pyridoxine has lacked efficacy in this setting. Although urea cream may mitigate any grade HFS, methylcobalamin specifically reduces grades that impact treatment adherence.
Limitations include restriction to Chinese patients, limiting generalisability, and the relatively short follow-up for survival assessment. The study did not evaluate biomarkers predictive of HFS risk or explore mechanistic endpoints such as skin biopsies. Future work should focus on wider population validation and mechanistic elucidation.
Conclusion
Oral methylcobalamin effectively and safely reduces the incidence of grade ≥2 hand-foot syndrome in women with HER2 negative early breast cancer receiving adjuvant capecitabine treatment. It offers a simple, well-tolerated prophylactic option to improve treatment adherence and quality of life. Clinicians should consider methylcobalamin supplementation in managing capecitabine-associated HFS, pending further validation in diverse populations and longer-term survival data.
Reference
Xia Y, Zhu Y, Ling L, Xu F, Yang Y, Ye J, Tan W, Chen Z, Liu Q, Wei W, Zhang J, Zhang A, Zhang L, Song E, Gong C. Effect of methylcobalamin on capecitabine induced hand-foot syndrome in patients with HER2 negative early breast cancer: multicentre, double blind, randomised, placebo controlled, phase 3 trial. BMJ. 2025 Sep 11;390:e084290. doi: 10.1136/bmj-2025-084290 IF: 42.7 Q1 . PMID: 40935571 IF: 42.7 Q1 ; PMCID: PMC12423938 IF: 42.7 Q1 .