Introduction
Emphysema, a major phenotype of chronic obstructive pulmonary disease (COPD), leads to destruction of lung tissue causing airway collapse, air trapping, and significant lung hyperinflation. Despite existing treatments, many patients with severe emphysema continue to experience high morbidity and mortality. Current interventions such as endobronchial valves, coils, and lung volume reduction surgery have limited availability, applicability, or durability, often dependent on emphysema heterogeneity and collateral ventilation. Persistent hyperinflation remains a critical therapeutic gap, as it directly contributes to debilitating dyspnea, decreased exercise capacity, impaired pulmonary function, and decreased quality of life.
Previous attempts to bypass expiratory airway collapse, including the Exhale Airway Stents for Emphysema (EASE) trial, demonstrated initial improvements but failed to sustain benefits due to stent occlusion and migration. A novel solution that offers durable airway patency and safely releases trapped air is urgently needed.
Study Design and Methods
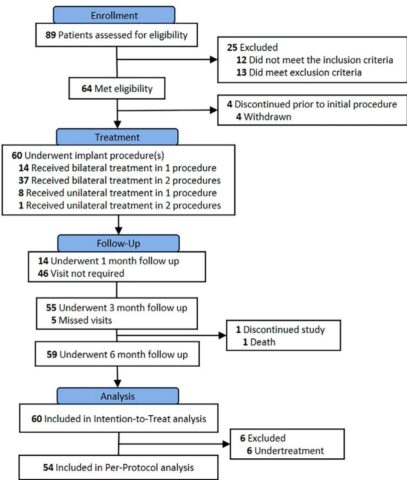
The BREATHE study is a pooled analysis of two prospective, multicenter, single-arm first-in-human trials (BREATHE-1 and BREATHE-2) conducted in Australia and Europe, respectively. Sixty patients with severe emphysema (FEV1 15–50% predicted, residual volume ≥180%, mMRC dyspnea scale ≥2) and evidence of significant emphysema on computed tomography (CT) were enrolled. Both patients with homogeneous and heterogeneous disease distributions, as well as varied lobar fissure completeness, were included to capture a broad representative cohort.
Participants underwent bronchoscopic implantation of up to six permanent self-expanding nitinol airway scaffolds (three per lung in bilateral disease), designed to connect emphysematous lung parenchyma with central bronchi, thereby promoting the release of trapped air and mitigating hyperinflation. The airway scaffold is a helix-shaped coil with a 10 mm unconstrained diameter and is available in lengths from 55 to 100 mm, compatible with existing therapeutic bronchoscopes. Implantation was conducted under general anesthesia with fluoroscopic and bronchoscopic guidance, and patients received prophylactic antibiotics and corticosteroids peri-procedurally.
Follow-up occurred at 1, 3, and 6 months, with outcome measures including safety assessment of serious adverse events (SAEs), technical feasibility of scaffold deployment, pulmonary function tests, patient-reported outcomes such as the St. George’s Respiratory Questionnaire for COPD Patients (SGRQ-C), COPD Assessment Test (CAT), mMRC dyspnea scale, 6-minute walk distance (6MWD), and CT imaging to assess airway patency and scaffold positioning.
Key Findings
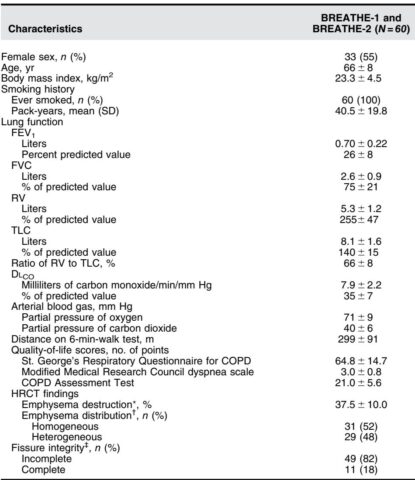
From May 2023 to October 2024, 98 implantation procedures were performed placing 328 airway scaffolds in 60 patients (55% female; mean age 66 ± 8 years; mean residual volume 255 ± 47% predicted). The procedure was technically successful in 92.4% of attempts, with most deployments described as having “no difficulty.”
Safety outcomes revealed 21.7% of patients experienced at least one device- or procedure-related SAE within 6 months. Pneumonia was the most common event (10%), followed by COPD exacerbation (5%). Importantly, no pneumothoraces occurred, contrasting favorably with the pneumothorax rates associated with endobronchial valve therapy. One death unrelated to the device occurred due to respiratory failure more than 3 months post-procedure.
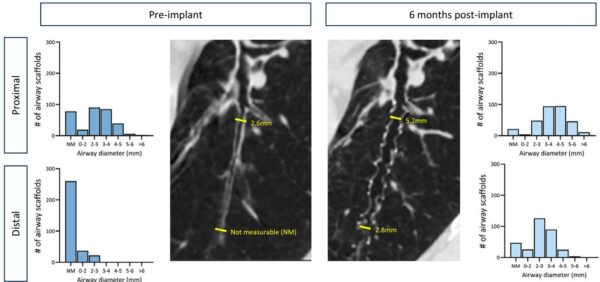
All airway scaffolds remained in situ at 6 months, and bronchoscopic and CT assessments demonstrated scaffold stability and preserved airway patency in the majority of treated airways. Quantitative CT showed increases in airway diameters at the scaffold sites compared to baseline, supporting the mechanical support effect of the scaffolds. Scaffold removals were infrequent (6.7% of patients) and were safely performed when indicated.
Significant and clinically meaningful improvements were observed in pulmonary function and patient symptoms. Residual volume (RV), a key measure of hyperinflation, decreased from baseline by a mean of 866 ml at 3 months and 753 ml at 6 months (both P < 0.0001). RV to total lung capacity (TLC) ratio, FEV1, and forced vital capacity (FVC) also improved significantly. Quality of life scores as measured by SGRQ-C and CAT, dyspnea scores (mMRC), and exercise capacity (6MWD) showed favorable changes exceeding their minimal clinically important differences. These improvements were consistent irrespective of emphysema distribution or fissure completeness.
Expert Commentary
The BREATHE trial represents a significant advance in the management of emphysema-related hyperinflation by introducing a durable, minimally invasive airway scaffold. Unlike prior stenting methods such as in the EASE trial, the nitinol scaffold’s flexible, helical design and low tissue contact density minimize tissue injury and foreign body reaction, preventing occlusion and migration. Preserved scaffold patency at 6 months establishes this approach as a promising alternative or adjunct to existing lung volume reduction therapies.
Although the trial lacked a randomized control and formal power calculations, the robust improvements in lung volumes, symptoms, and exercise capacity align with clinically meaningful benefits seen in approved therapies. The absence of pneumothorax marks an improved safety profile compared to endobronchial valves, opening the possibility for broader patient applicability, including those without favorable fissure anatomy or with homogeneous emphysema.
Future studies should focus on long-term durability beyond 6 months, randomized controlled evaluations, and potential optimization of patient selection and scaffold deployment strategies to maximize clinical outcomes.
Conclusion
The BREATHE study demonstrates for the first time the feasibility, safety, and initial efficacy of self-expanding nitinol airway scaffolds to relieve hyperinflation in severe emphysema. This novel bronchoscopic intervention safely reduces air trapping, enhances lung function, and improves quality of life and exercise capacity with a favorable safety profile at six months. These encouraging results warrant further investigation in larger, controlled trials to confirm clinical benefits and define its role within emphysema management.
References
Tana A, Valipour A, Ing A, Steinfort DP, Orton CM, Klooster K, Klemm T, Williamson JP, Christie JJ, Garner JL, Koster TD, Welz K, van Dijk M, Mayse ML, Shah PL, Slebos DJ; BREATHE Study Group. Airway Scaffolds for Emphysema-related Hyperinflation: Six-Month Results from the BREATHE Trial. Am J Respir Crit Care Med. 2025 Jul;211(7):1175-1184. doi: 10.1164/rccm.202502-0378OC IF: 19.4 Q1 . PMID: 40387356 IF: 19.4 Q1 ; PMCID: PMC12264656 IF: 19.4 Q1 .