Highlight
Lubiprostone administration in CKD patients preserved estimated glomerular filtration rate (eGFR) and slowed renal decline over 24 weeks without altering uremic toxin levels.
Multiomics analyses revealed lubiprostone increased gut microbial agmatine deiminase (aguA) expression, resulting in elevated spermidine, a polyamine beneficial for mitochondrial function.
Experimental models demonstrated that spermidine administration improved mitochondrial morphology, bioenergetics, and reduced inflammation in renal failure.
Lubiprostone was well tolerated with manageable gastrointestinal side effects, highlighting its safety
Introduction
nChronic kidney disease (CKD) poses a major global health challenge with a rising burden of morbidity and mortality. Despite advances in pharmacotherapy using agents like ACE inhibitors, ARBs, SGLT2 inhibitors, MR antagonists, and GLP-1 agonists, CKD progression to end-stage renal disease remains substantial. Constipation, highly prevalent among CKD patients, independently predicts accelerated decline in kidney function. The pathophysiology linking constipation with CKD progression involves gut microbiota dysbiosis, increased intestinal permeability (“leaky gut”), bacterial translocation, elevated uremic toxins, and systemic inflammation.
Emerging therapies targeting colonic transit and gut microbiota, such as novel laxatives including lubiprostone, present potential for modifying the gut-kidney axis. Previous animal studies showed that lubiprostone reduces circulating gut-derived uremic toxins and improves renal outcomes. However, evidence in human CKD remains sparse, and concerns about safety—such as dehydration from diarrhea—have limited clinical application. This phase 2 randomized, double-blind, placebo-controlled trial was designed to evaluate lubiprostone’s effects on uremic toxins, renal function, and safety in CKD patients and to explore mechanistic insights through integrated multiomics analyses.
Study Design
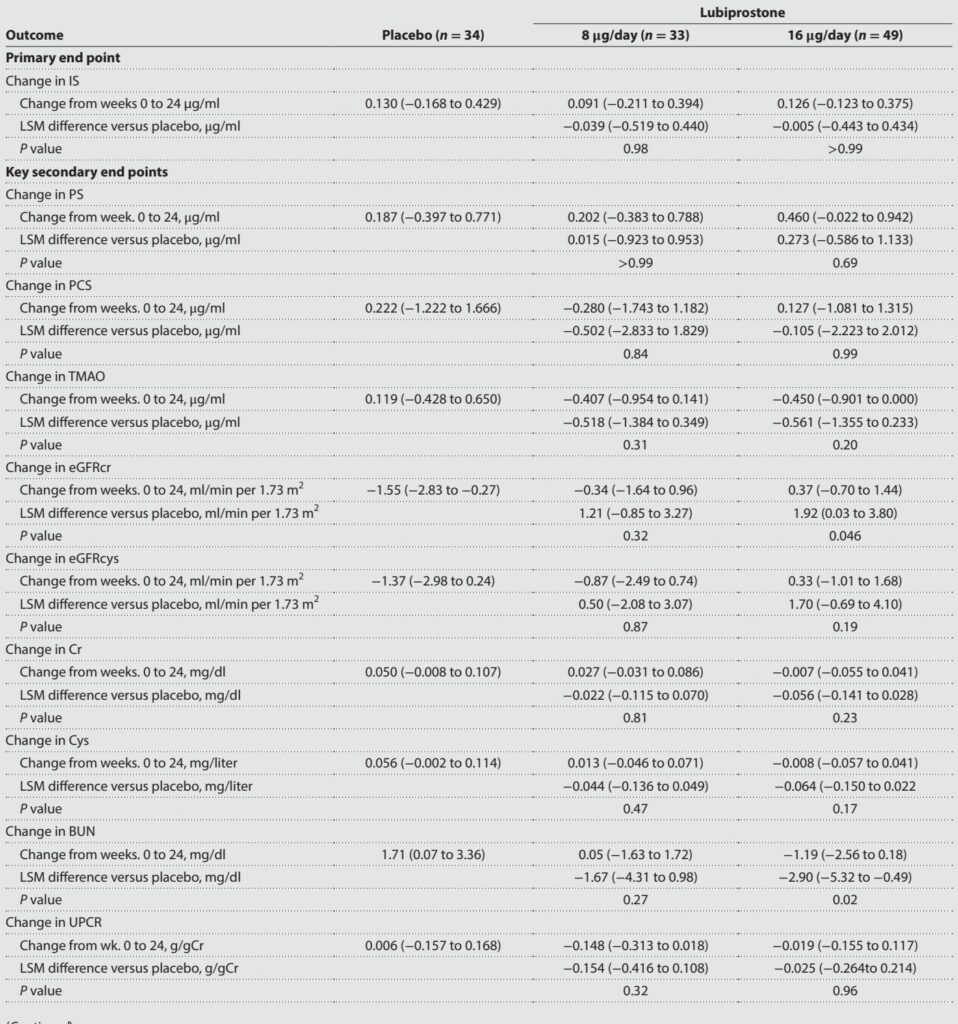
This multicenter trial was conducted across nine sites in Japan, enrolling 118 adult patients with stage IIIb to IV CKD (eGFR 25–45 mL/min/1.73 m²). Participants were randomized in a 2:2:3 ratio to receive daily lubiprostone 8 µg, lubiprostone 16 µg, or placebo for 24 weeks. The primary endpoint was change in serum indoxyl sulfate (IS), a key gut-derived uremic toxin, at 24 weeks compared to baseline. Secondary endpoints included changes in other uremic toxins (phenyl sulfate, p-cresyl sulfate, trimethylamine N-oxide), renal function markers (eGFR estimated by creatinine and cystatin C, blood urea nitrogen, creatinine slope, urinary protein-creatinine ratio), and safety assessments.
Comprehensive multiomics profiling—including capillary electrophoresis time-of-flight mass spectrometry (CE-TOFMS), 16S rRNA gene sequencing, shotgun metagenomics, and RNA sequencing—was performed to elucidate gut microbial and metabolic alterations associated with lubiprostone treatment. Animal experiments in adenine-induced renal failure mice assessed effects of spermidine (SPD), a polyamine identified in human studies, on renal mitochondrial function.
Key Findings
Primary analyses showed that lubiprostone treatment did not significantly reduce serum IS or other uremic toxins compared to placebo after 24 weeks. However, notable preservation of renal function was observed in the 16 µg lubiprostone group, demonstrated by stabilized eGFR-cr levels and improved slopes of reciprocal creatinine and eGFR. Subgroup analyses revealed greater renoprotection in patients with moderate (eGFR 36–45) compared to severe CKD. No significant changes occurred in eGFR estimated by cystatin C or urinary protein excretion, indicating a selective functional effect.
Safety data confirmed a tolerable profile; mild-to-moderate diarrhea occurred in up to 16% of lubiprostone-treated patients but did not lead to significant electrolyte disturbances or dehydration.
Multiomics analyses elucidated mechanisms underpinning renoprotection. Lubiprostone altered gut microbiota composition, increasing beneficial genera such as Roseburia, Blautia, and Marvinbryantia—known short-chain fatty acid producers—and decreasing Desulfovibrio. Functional metagenomics revealed elevation of agmatine deiminase (aguA) gene abundance, pivotal for microbial polyamine synthesis.
Subsequent metabolomic profiling demonstrated increased fecal lactate and decreased ornithine, a polyamine precursor, suggesting accelerated polyamine biosynthesis. Targeted quantification revealed elevated plasma spermidine levels in lubiprostone responders, linking microbial metabolic shifts to host systemic bioactive polyamines.
Responder analyses indicated that patients exhibiting improvements in renal function post-lubiprostone had differential baseline gut microbiota profiles characterized by increased aguA-containing bacteria and decreased inflammatory species like Holdemania.
Animal studies administering spermidine in renal failure mice replicated renoprotective effects: plasma creatinine was reduced, renal tubular pathology ameliorated, and plasma GDF15—a marker of mitochondrial stress—decreased. Advanced microscopy of proximal tubular mitochondria showed preservation of mitochondrial volume, network integrity, and reduced fragmentation after spermidine treatment.
In vitro, spermidine enhanced mitochondrial respiration, spare respiratory capacity, ATP production, and glycolytic flux in human proximal tubular HK-2 cells, indicating improved bioenergetic function. RNA sequencing of mouse kidneys revealed that spermidine downregulated proinflammatory cytokine genes and restored mitochondrial gene expression, especially oxidative phosphorylation components.
Expert Commentary
This trial challenges the conventional paradigm that direct reduction of uremic toxins is the principal mechanism to attenuate CKD progression. Despite unaltered toxin levels, lubiprostone demonstrated clinical benefit, highlighting mitochondrial function restoration via microbe-derived polyamines as a novel therapeutic axis.
Spermidine’s role in promoting autophagy, reducing oxidative stress, and preserving mitochondrial integrity aligns with mounting evidence linking cellular bioenergetics to renal health. The gut microbiota’s capacity to modulate host polyamine pools adds an important dimension to CKD pathophysiology and treatment. Lubiprostone’s known pro-motility effect may further enhance colonic transit, facilitating microbiome remodeling.
Limitations include a relatively short 24-week duration and modest sample size, warranting confirmation in larger, longer-term studies. Moreover, the study population was exclusively Asian, potentially limiting generalizability. Discrepancies between creatinine- and cystatin C-based eGFR suggest complex influences on renal function metrics, meriting further investigation.
Conclusion
Lubiprostone offers a promising new therapeutic approach in CKD, demonstrating renoprotective effects independent of direct uremic toxin reduction. By modulating the gut microbiota and enhancing systemic polyamine levels, particularly spermidine, lubiprostone improves mitochondrial function and mitigates renal decline. This integrative gut-kidney axis opens avenues for innovative CKD management targeting microbial metabolism and mitochondrial health. Larger clinical trials focused on hard renal outcomes and exploration of surrogate markers such as aguA and plasma spermidine are encouraged to translate these findings into clinical practice.
References
1. Watanabe S, Nakayama M, Yokoo T, Sanada S, Ubara Y, Komatsuda A, Asanuma K, Suzuki Y, Konta T, Kazama JJ, Suzuki T, Fukuda S, et al. Lubiprostone in chronic kidney disease: Insights into mitochondrial function and polyamines from a randomized phase 2 clinical trial. Sci Adv. 2025 Aug 29;11(35):eadw3934.
2. Sumida K, Molnar MZ, Potukuchi PK, Thomas F, Lu JL, Yamagata K, Kalantar-Zadeh K, Kovesdy CP. Constipation and incident CKD. J Am Soc Nephrol. 2017;28:1248–1258.
3. Mishima E, Fukuda S, Shima H, Hirayama A, Akiyama Y, Takeuchi Y, Fukuda NN, et al. Alteration of the intestinal environment by lubiprostone is associated with amelioration of adenine-induced CKD. J Am Soc Nephrol. 2015;26:1787–1794.
4. Kikuchi K, Saigusa D, Kanemitsu Y, Matsumoto Y, Thanai P, Suzuki N, et al. Gut microbiome-derived phenyl sulfate contributes to albuminuria in diabetic kidney disease. Nat Commun. 2019;10:1835.
5. Hofer SJ, Liang Y, Zimmermann A, Schroeder S, Dengjel J, Kroemer G, et al. Spermidine-induced hypusination preserves mitochondrial and cognitive function during aging. Autophagy. 2021;17:2037–2039.
6. Levey AS, Gansevoort RT, Coresh J, Inker LA, Heerspink HL, Grams ME, et al. Change in Albuminuria and GFR as end points for clinical trials in early stages of CKD: A scientific workshop sponsored by the National Kidney Foundation. Am J Kidney Dis. 2020;75:84–104