Highlight
- The BRADiNP randomized crossover trial evaluated fulmetibant in diabetic neuropathic pain (DNP), showing no significant analgesic benefit over placebo.
- Despite preclinical promise, the 450 mg once-daily fulmetibant regimen did not meaningfully reduce 24-hour average pain intensity in patients with painful distal symmetric sensorimotor neuropathy.
- Adverse events were mostly mild or moderate, occurring in 41.8% of patients on fulmetibant versus 32.9% on placebo, indicating a manageable safety profile.
- The study underscores the ongoing unmet need for effective therapies in managing DNP and challenges in translating molecular targets into clinical treatments.
Study Background and Disease Burden
Diabetic neuropathic pain (DNP) is a common and debilitating complication affecting up to 50% of patients with long-standing diabetes mellitus, encompassing both type 1 and type 2 diabetes mellitus. DNP is characterized by painful distal symmetric sensorimotor neuropathy, causing persistent burning, stabbing, and electric shock-like pain predominantly in the lower extremities. This chronic pain substantially impairs quality of life, contributes to sleep disturbances, and challenges effective management. Current pharmacotherapies—including anticonvulsants, antidepressants, and opioids—often provide insufficient relief and are commonly associated with significant side effects.
Bradykinin, a proinflammatory peptide, is implicated in neuropathic pain pathways through its action on bradykinin-1 receptors (B1R), which are upregulated in chronic inflammation and nerve injury. Preclinical models demonstrated that B1R antagonism could attenuate neuropathic pain, positioning fulmetibant, a selective B1R antagonist, as a promising novel analgesic candidate for DNP.
Study Design
The BRADiNP study (ClinicalTrials.gov NCT05219812) was a multicenter, randomized, double-blind, placebo-controlled, two-treatment complete crossover phase 2a trial. The study enrolled adults with either type 1 or type 2 diabetes mellitus who had painful distal symmetric sensorimotor neuropathy of more than six months’ duration and measurable neuropathic pain. After a blinded washout period removing prior DNP treatments, participants were randomized 1:1 to receive either fulmetibant 450 mg once daily or placebo for four weeks, followed by a crossover to the alternate treatment for another four weeks.
The primary efficacy endpoint was the change from baseline in the weekly mean 24-hour average pain intensity score measured at week 4 of each treatment period, using validated pain scales. Secondary endpoints included safety and tolerability assessments based on reported adverse events and laboratory parameters.
Key Findings
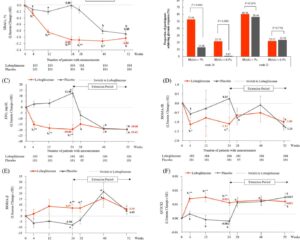
A total of 79 participants started treatment with fulmetibant and 79 with placebo; 75 completed both treatment periods. At the primary endpoint (week 4), the mean treatment difference in pain intensity between fulmetibant and placebo was 0.07 with a 95% confidence interval ranging from -0.170 to 0.314, indicating no statistically or clinically significant analgesic benefit of fulmetibant over placebo.
The absence of efficacy was consistent across subgroups and secondary measures. Regarding safety, 41.8% of participants reported adverse events while on fulmetibant, compared to 32.9% on placebo. Most adverse events were mild to moderate, including fatigue, dizziness, and gastrointestinal symptoms, with no serious adverse events attributed to fulmetibant.
These findings suggest that, despite the theoretical rationale and preclinical evidence supporting B1R blockade for neuropathic pain, fulmetibant at the tested dose and regimen did not provide meaningful therapeutic benefit for patients with DNP.
Expert Commentary
The BRADiNP study offers critical insights into the challenges of translating molecular-targeted treatments from bench to bedside in complex neuropathic pain conditions. As noted by neuropathic pain specialists, diabetic neuropathy involves multifactorial pathophysiology including metabolic, vascular, and neuroimmune components that may not be sufficiently addressed by B1R antagonism alone.
Potential reasons for the negative outcome include insufficient central nervous system penetration of fulmetibant, compensatory pro-nociceptive pathways, and patient heterogeneity in pain mechanisms. The study’s crossover design strengthens internal validity by reducing interindividual variability, but a four-week treatment period might have been too brief to observe maximal therapeutic effects.
Future research could explore combination therapies, alternative targets within the kinin–kallikrein system, or stratified patient selection based on biomarker profiles. Moreover, rigorous mechanistic studies may help clarify why B1R inhibition failed to replicate preclinical analgesic efficacy in human DNP.
Conclusion
The BRADiNP phase 2a trial demonstrated that fulmetibant, a bradykinin-1 receptor antagonist, did not significantly reduce neuropathic pain intensity in patients with painful diabetic neuropathy compared with placebo. While it was generally well tolerated, the lack of analgesic efficacy highlights the complexity of DNP pathogenesis and the limitations of single-target approaches.
These results emphasize the urgent need for continued innovation and deeper mechanistic understanding to identify effective, safe treatments for DNP. Clinicians should remain guided by current evidence-based neuropathic pain management strategies while remaining attentive to emerging novel agents undergoing robust clinical evaluation.
References
1. Stemper B, Löwen S, Fritsch A, Hoffmann A, Sarkar A. The bradykinin-1 receptor antagonist fulmetibant in patients with diabetic neuropathic pain: the randomized, crossover, placebo-controlled, double-blind, phase 2a BRADiNP study. Pain. 2025 Oct 1;166(10):2421-2429. doi: 10.1097/j.pain.0000000000003642. Epub 2025 May 6. PMID: 40334047.
2. Ziegler D, Fonseca V. From guideline to patient: a review of recent recommendations for pharmacotherapy of painful diabetic neuropathy. J Diabetes Complications. 2015;29(1):146-156.
3. Fields HL. Mechanisms of neuropathic pain: therapeutic implications. Rev Neurol Dis. 2006;3(4):186-193.
4. Kemp TI, Kingsbury L, McNair K, Graham J, Helyes Z, Szabo A. Bradykinin and neuropathic pain: a novel pharmacological target? Eur J Pain. 2017;21(9):1431-1442.