Highlight
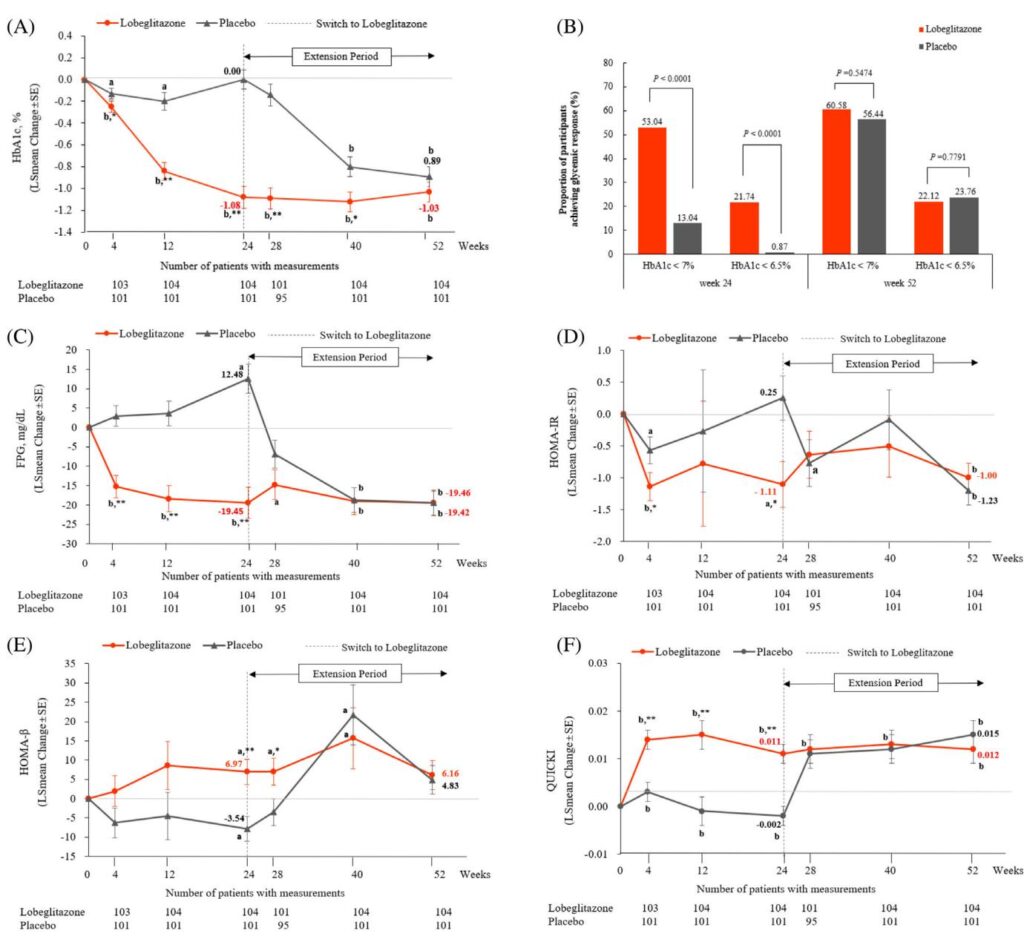
- Adding lobeglitazone to metformin and sitagliptin significantly reduced HbA1c by approximately 1% at 24 weeks, sustained through 52 weeks.
- Improvements included better insulin sensitivity (HOMA-IR), enhanced pancreatic β-cell function (HOMA-β), and favorable changes in lipid profiles.
- The safety profile was comparable to placebo with mild adverse events such as manageable edema and modest weight gain.
- This triple therapy approach offers a valuable option for Asian patients with type 2 diabetes inadequately controlled with metformin and DPP-4 inhibitors.
Background
Type 2 diabetes mellitus (T2DM) is characterized by hyperglycaemia predominantly caused by insulin resistance and pancreatic β-cell dysfunction. Despite initial dual therapy with metformin and dipeptidyl peptidase-4 (DPP-4) inhibitors such as sitagliptin, many patients struggle to reach glycaemic targets, necessitating additional pharmacologic agents. While thiazolidinediones (TZDs) like pioglitazone are potent insulin sensitizers targeting PPAR-γ, their use has declined due to safety concerns involving cardiovascular events and cancer. Lobeglitazone, a novel TZD with an improved safety profile, has shown promising efficacy and tolerability in preclinical and earlier clinical trials. Given the rising prevalence of non-obese and elderly Asian patients with T2DM and the established superiority of triple combination therapies, this study evaluates the addition of lobeglitazone to metformin and sitagliptin dual therapy for improved glycaemic control and metabolic benefits in Korean patients inadequately controlled on dual therapy.
Study Design
This phase III, randomized, double-blind, placebo-controlled, multicentre trial was conducted across 19 centers in the Republic of Korea. A total of 231 adults with T2DM inadequately controlled (HbA1c 7.0%–10.0%) on stable doses of metformin (≥1000 mg/day) and sitagliptin (100 mg/day) were randomized (1:1) to either lobeglitazone 0.5 mg daily or placebo for 24 weeks. This was followed by a 28-week open-label extension where all participants received lobeglitazone. Patients maintained their original metformin and sitagliptin doses, along with standardized diet and exercise regimens. Key exclusion criteria comprised type 1 diabetes, recent cardiovascular events, and active malignancy.
The primary endpoint was the change in glycated haemoglobin (HbA1c) at week 24. Secondary endpoints included changes in fasting plasma glucose (FPG), insulin sensitivity and β-cell function assessed by HOMA-IR, HOMA-β, QUICKI, lipid parameters (total cholesterol, LDL-C, HDL-C, small dense LDL-C, triglycerides, free fatty acids), and safety assessments.
Key Findings
At 24 weeks, lobeglitazone treatment resulted in a significant mean HbA1c decline of -1.00% (±0.09) versus placebo (+0.02% ±0.09), with an adjusted mean difference of -1.03% (95% CI, -1.23 to -0.82, p<0.0001). This glycaemic improvement was progressive throughout the treatment period and sustained up to week 52 during the open-label phase.
The proportion of patients achieving target HbA1c <7.0% was markedly higher in the lobeglitazone group (53.04%) compared with placebo (13.04%) at week 24 (p<0.0001). Similarly, HbA1c <6.5% was attained by 27.14% in the lobeglitazone group versus 0.87% in placebo (p<0.0001).
Significant reductions in FPG (adjusted mean difference -30.60 mg/dL, p<0.0001) were observed with lobeglitazone. Indicators of insulin resistance and sensitivity improved substantially: HOMA-IR decreased indicating enhanced insulin sensitivity, HOMA-β increased reflecting β-cell functional improvement, and QUICKI improved, all with highly significant differences from placebo (p<0.0001).
Lipid profiles also showed beneficial changes. Small dense LDL-C decreased significantly at 52 weeks by 1.46% from baseline (p=0.0003), while HDL-C showed an increasing trend. Free fatty acids decreased, contributing to an improved atherogenic lipid profile. These changes were independent of concomitant lipid-lowering therapies.
In terms of safety, overall adverse event (AE) rates during the initial 24-week period were similar between lobeglitazone (27.6%) and placebo (30.4%). Most AEs were mild or moderate. Edema, a common TZD-related side effect, occurred in 3.45% of lobeglitazone-treated patients, all mild except one moderate eyelid edema. Weight gain averaged 1.69 kg in the lobeglitazone group versus weight loss in placebo, consistent with TZD class effects. No cases of heart failure, fractures, or liver enzyme elevations were reported during the study.
Expert Commentary
This robust phase III trial underscores the effectiveness of lobeglitazone as an add-on therapy to metformin and sitagliptin in Asians with inadequately controlled T2DM. The 1% HbA1c reduction aligns favorably with triple therapies involving SGLT-2 inhibitors and DPP-4 inhibitors, suggesting lobeglitazone offers a potent alternative, particularly where SGLT-2 inhibitors may be less favored due to population characteristics.
The improvements in insulin resistance and β-cell function highlight lobeglitazone’s mechanistic advantages in addressing core pathophysiological defects in T2DM. The positively altered lipid profile, especially the reduction of small dense LDL-C, suggests potential cardiovascular benefits warranting long-term investigation.
Weight gain and edema remain concerns typical of TZDs; however, their mild incidence and absence of serious cardiovascular safety issues in this study reinforce lobeglitazone’s improved tolerability. The study’s strength includes its rigorous design, comprehensive metabolic assessments, and 52-week follow-up.
Limitations include that the study population was exclusively Korean, potentially affecting generalizability. Long-term cardiovascular outcome data are needed to definitively establish cardiovascular safety and benefits.
Conclusion
Addition of lobeglitazone to ongoing metformin and sitagliptin therapy in patients with type 2 diabetes leads to clinically meaningful and sustained glycaemic improvement, enhanced insulin sensitivity, better β-cell function, and improved atherogenic lipid profile, with a tolerable safety profile. This triple oral regimen represents a viable and beneficial therapeutic option for Asian patients inadequately controlled on dual oral therapy. Further studies should assess long-term cardiovascular outcomes and applicability in diverse populations.
References
1. Hur KY, Moon MK, Park JS, et al. 2021 Clinical practice guidelines for diabetes mellitus of the Korean Diabetes Association. Diabetes Metab J. 2021;45(4):461-481.
2. Han S, Iglay K, Davies MJ, Zhang Q, Radican L. Glycemic effectiveness and medication adherence with fixed-dose combination or coadministered dual therapy of antihyperglycemic regimens: a meta-analysis. Curr Med Res Opin. 2012;28(6):969-977.
3. American Diabetes Association Professional Practice Committee. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2024. Diabetes Care. 2024;47(7):S158-S178.
15. Kim SG, Kim DM, Woo JT, et al. Efficacy and safety of lobeglitazone monotherapy in patients with type 2 diabetes mellitus over 24-weeks: a multicenter, randomized, double-blind, parallel-group, placebo controlled trial. PLoS One. 2014;9:e92843.
16. Kwon MJ, Lee YJ, Jung HS, et al. The direct effect of lobeglitazone, a new thiazolidinedione, on pancreatic beta cells: a comparison with other thiazolidinediones. Diabetes Res Clin Pract. 2019;151:209-223.
17. Kim SH, Kim SG, Kim DM, et al. Safety and efficacy of lobeglitazone monotherapy in patients with type 2 diabetes mellitus over 52 weeks: an open-label extension study. Diabetes Res Clin Pract. 2015;110(3):e27-e30.
23. Qaseem A, Obley AJ, Shamliyan T, et al. Newer pharmacologic treatments in adults with type 2 diabetes: a clinical guideline from the American College of Physicians. Ann Intern Med. 2024;177(5):658-666.
31. Kim BY, Kwon HS, Kim SK, et al. A real-world study of long-term safety and efficacy of Lobeglitazone in Korean patients with type 2 diabetes mellitus. Diabetes Metab J. 2022;46(6):855-865.
35. Jin ES, Shim JS, Kim SE, et al. Dyslipidemia fact sheet in South Korea, 2022. Diabetes Metab J. 2023;47(5):632-642.