Highlight
- Paclitaxel-coated devices did not improve disease-specific quality of life at 1 year in patients with intermittent claudication undergoing infrainguinal endovascular revascularisation.
- Long-term mortality over a median of 7.1 years was not significantly different; however, 5-year mortality was higher in the paclitaxel-coated device group compared to uncoated devices.
- The study’s findings challenge the routine use of paclitaxel-coated devices in this patient population given the lack of quality of life benefit and potential mortality risk.
- The SWEDEPAD 2 trial employed a pragmatic, multicentre, registry-based randomized controlled design with robust follow-up data on over 1100 patients.
Study Background and Disease Burden
Peripheral artery disease (PAD) is a prevalent and progressive condition characterized by atherosclerotic occlusion of arteries supplying the lower extremities, resulting in ischemic symptoms such as intermittent claudication. Claudication limits patient mobility and quality of life due to exertional leg pain. Infrainguinal endovascular revascularisation using balloons and stents is a common approach to restore blood flow in femoropopliteal segments.
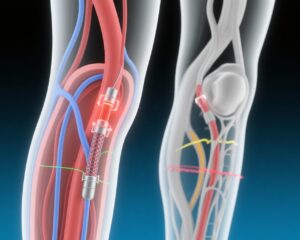
Drug-coated devices, particularly those coated with paclitaxel, have been developed to reduce restenosis rates by inhibiting neointimal hyperplasia. Despite widespread adoption, evidence on whether these devices confer meaningful improvements in patient-centred outcomes such as disease-specific quality of life or reduce mortality remains fragmented and inconclusive. Some prior meta-analyses raised concerns about increased late mortality associated with paclitaxel-coated devices, further heightening the need for rigorous prospective trials.
Study Design
The Swedish Drug-Elution Trial in Peripheral Arterial Disease 2 (SWEDEPAD 2) was a pragmatic, participant-masked, registry-based, randomized controlled trial conducted across 22 Swedish vascular centres over nine years (Nov 2014 – Sept 2023). Eligible were adults aged 18 years or older with intermittent claudication defined by Rutherford categories 1-3 undergoing planned infrainguinal endovascular intervention. Key exclusions included acute thromboembolic or aneurysmal infrainguinal disease.
After successful guidewire crossing, participants were randomized (1:1) to receive either paclitaxel-coated balloons or stents or equivalent uncoated devices, stratified by centre with concealed allocation through a secure web-based registry system. The primary efficacy endpoint was patient-perceived quality of life at 1 year measured by the Vascular Quality of Life Questionnaire-6 (VascuQoL-6), a validated PAD-specific instrument evaluating symptoms, activities, and social aspects.
Secondary endpoints included long-term all-cause mortality assessed over a median follow-up of 7.1 years. The trial aimed to balance pragmatic applicability with rigorous randomized methodology, leveraging Sweden’s comprehensive vascular registries and high follow-up completeness (98.3%).
Key Findings
A total of 1155 patients were randomized: 577 to paclitaxel-coated and 578 to uncoated devices; 1136 (98.3%) had follow-up data for analysis. The median age was 73 years, with a balanced sex distribution and 33.7% having diabetes. Most presented with severe claudication (Rutherford 3) and underwent femoropopliteal interventions (96.1%).
At 1 year, no significant difference in disease-specific quality of life was observed between groups — mean VascuQoL-6 scores differed by only -0.02 (95% CI -0.66 to 0.62; p=0.96), indicating no benefit from paclitaxel-coated devices in symptom relief or functional status.
Regarding safety, all-cause mortality was statistically similar over the total follow-up period (HR 1.18; 95% CI 0.94-1.48; p=0.16). However, analysis of the 5-year window showed a significantly higher incidence of death in the paclitaxel group (4.57 vs 3.28 per 100 person-years), with a hazard ratio of 1.47 (95% CI 1.09-1.98; p=0.010), consistent with previous meta-analytic safety concerns.
Other important findings include similar procedural success and complication rates, although the study’s main focus emphasized patient-centred quality of life and long-term mortality endpoints.
Expert Commentary
The SWEDEPAD 2 trial addresses a critical clinical controversy sparked by earlier meta-analyses suggesting late mortality risk after use of paclitaxel-coated devices. Notably, this trial underscores that despite theoretical antiproliferative advantages, these devices do not translate into improved quality of life for patients with intermittent claudication undergoing infrainguinal revascularisation.
The increased 5-year mortality observed warrants cautious interpretation. Biological plausibility remains debated, as paclitaxel’s localized drug delivery might pose systemic risks, especially in frail elderly populations with cardiovascular comorbidities. While the absolute risk increase is modest, patients, clinicians, and guideline committees should consider these findings in shared decision-making and device selection.
Limitations of SWEDEPAD 2 include lack of blinding of treating clinicians (participant-masked only), potential residual confounding despite randomization, and not fully dissecting causes of death, which could illuminate mechanistic risk pathways. However, the trial’s pragmatic design and comprehensive registry follow-up amplify its external validity.
Current peripheral arterial disease guidelines may need revision to reflect these nuanced safety and efficacy data, particularly advocating for personalized approaches rather than routine use of paclitaxel-coated devices in intermittent claudication.
Conclusion
The SWEDEPAD 2 trial provides robust evidence that paclitaxel-coated devices offer no quality of life benefit at 1 year compared to uncoated devices in patients with Rutherford stage 1-3 peripheral artery disease undergoing infrainguinal endovascular revascularisation. Furthermore, the significant increase in 5-year mortality incidence linked to paclitaxel-coated devices raises important safety concerns that do not support their routine use in this population.
These findings highlight the necessity for careful patient selection, transparent risk-benefit discussion, and continued research into safer and more effective technologies for lower limb revascularisation. Future investigations should focus on mechanistic understanding of paclitaxel systemic effects and alternative drug coatings or device technologies.
References
- Nordanstig J, James S, Andersson M, et al; SWEDEPAD trial investigators. Paclitaxel-coated versus uncoated devices for infrainguinal endovascular revascularisation in patients with intermittent claudication (SWEDEPAD 2): a multicentre, participant-masked, registry-based, randomised controlled trial. Lancet. 2025 Sep 13;406(10508):1115-1127. doi:10.1016/S0140-6736(25)01584-3. PMID: 40902614.
- Brodmann M, Krishnan P, Bausback Y, et al. Drug-Coated Balloons Versus Standard Percutaneous Transluminal Angioplasty for the Treatment of Superficial Femoral and Popliteal Peripheral Arterial Disease: Long-Term Outcomes of the ILLUMENATE Pivotal Trial. Circ Cardiovasc Interv. 2018;11(2):e005891.
- Katsanos K, Spiliopoulos S, Kitrou P, et al. Risk of Death Following Application of Paclitaxel-Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc. 2018 Dec 18;7(24):e011245.