Highlight
- Bexotegrast, an oral dual inhibitor of integrins $b1_vb26$ and $b1_vb21$, was well tolerated in IPF patients over 12 weeks.
- Compared with placebo, bexotegrast treatment was associated with reduced forced vital capacity (FVC) decline, indicating potential antifibrotic efficacy.
- Quantitative lung fibrosis (QLF) imaging and circulating biomarkers ITGB6 and PRO-C3 showed dose-dependent improvement with bexotegrast.
- The drug’s favorable safety profile supports ongoing longer-term trials to establish durability of benefit.
Background
Idiopathic pulmonary fibrosis (IPF) is a progressive, fatal lung disorder characterized by excessive fibrosis that impairs respiratory function, manifesting as worsening cough and dyspnea. Median survival without treatment is 3 to 4 years. Current approved therapies—pirfenidone and nintedanib—slow but do not halt disease progression and often have tolerability issues such as gastrointestinal adverse effects. Thus, there remains an urgent unmet need for safer and more effective treatments.
Activation of transforming growth factor-beta (TGF-b2), a principal driver of fibrogenesis, occurs via the integrins $b1_vb26$ and $b1_vb21$, which are upregulated in IPF lungs. Targeting these integrins to block local TGF-b2 activation represents a novel antifibrotic strategy. Bexotegrast (PLN-74809) is an oral, once-daily small molecule that selectively inhibits these integrins, preventing the profibrogenic cascade initiated by TGF-b2.
Study Design
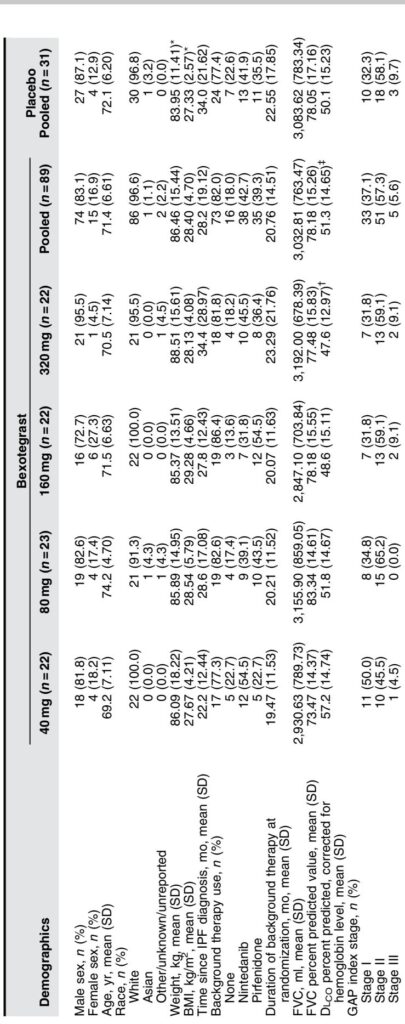
The INTEGRIS-IPF trial (NCT04396756) was a Phase-2a, randomized, double-blind, placebo-controlled, dose-ranging study conducted at 39 sites internationally. A total of 119 participants aged predominantly in their seventies with confirmed IPF were randomized 3:1 to receive daily oral bexotegrast at doses of 40 mg, 80 mg, 160 mg, or 320 mg or placebo for a minimum of 12 weeks. Background IPF therapies (pirfenidone or nintedanib) were permitted if stable for over 3 months prior to enrollment; approximately 80% of participants were on background treatment.
The primary endpoint focused on safety, specifically the incidence of treatment-emergent adverse events (TEAEs). Exploratory efficacy endpoints included change from baseline to week 12 in forced vital capacity (FVC), quantitative lung fibrosis extent assessed by high-resolution computed tomography (HRCT), and fibrosis-related circulating biomarkers including integrin beta-6 (ITGB6) and type III collagen synthesis neoepitope (PRO-C3).
Key Findings
Safety and Tolerability: Bexotegrast was well tolerated across all doses with no dose-dependent increase in TEAEs. The overall incidence of TEAEs was comparable between bexotegrast (69.7%) and placebo (67.7%) arms. The most common adverse event was diarrhea, predominantly occurring in participants also receiving nintedanib, consistent with known effects of that drug. No treatment-related serious adverse events or deaths were reported. Laboratory, vital signs, and ECG assessments showed no safety signals.
Pharmacokinetics: Bexotegrast exposure increased approximately proportionally with dose, supporting predictable pharmacokinetics.
Efficacy: In the modified intent-to-treat population (with over 80% on background therapy), bexotegrast significantly reduced FVC decline compared with placebo at 80 mg and 320 mg doses at 12 weeks. The placebo group experienced an average FVC decline of 20bc110.5 ml20bc, while bexotegrast groups showed smaller declines or modest improvement (for example, +29.4 ml with 320 mg). This reduction in lung function loss was observed regardless of background therapy use.
Dose-dependent reductions were also seen in the proportion of participants experiencing 20bc10% relative or absolute decline in FVC percent predicted. HRCT-based quantitative lung fibrosis extent demonstrated stabilization or limited progression at 160 mg and 320 mg doses relative to placebo. Biomarker analyses revealed dose-dependent decreases in plasma ITGB6 and serum PRO-C3 levels, both implicated in the fibrotic process and predictive of disease progression.
In an extension cohort, a majority (88.9%) of patients on 320 mg who showed FVC improvement at 12 weeks maintained it at 24 weeks, unlike placebo recipients.
Expert Commentary
The INTEGRIS-IPF trial provides promising early clinical evidence that bexotegrast can safely inhibit integrin-mediated TGF-b2 activation and reduce fibrotic progression in IPF. Unlike previous integrin-blocking antibodies that raised concerns about increased acute exacerbation risk, bexotegrast was not associated with such safety signals, possibly reflecting differences in drug modality and pharmacodynamics.
The study’s inclusion of participants on stable background antifibrotic treatment reflects real-world clinical practice, and findings suggest bexotegrast may add benefit without additional toxicity. However, the study’s relatively short duration (12 weeks) and modest sample size limit definitive conclusions about long-term efficacy and survival impact. The observed variable FVC changes underscore the inherent measurement variability in short-term pulmonary function testing.
The biomarker and imaging data bolster the biological plausibility of bexotegrast’s antifibrotic action. ITGB6 reduction aligns with blockade of targeted integrins, and PRO-C3 changes reflect modulation of collagen turnover.
Future larger and longer trials, such as the ongoing 52-week BEACON-IPF study, are essential to confirm sustained benefit and characterize clinical endpoints including mortality, quality of life, and exacerbation rates. Enrollment should also strive for more ethnically diverse populations to enhance generalizability.
Conclusion
Bexotegrast demonstrates a favorable safety profile and encouraging signals of efficacy in reducing lung function decline and fibrosis progression in patients with idiopathic pulmonary fibrosis. By targeting key integrins to inhibit TGF-b2 activation, bexotegrast offers a novel mechanism that may complement existing antifibrotic therapies. Ongoing and future Phase 3 studies will be pivotal to determine its role in clinical practice and its potential to improve outcomes in this devastating disease.
References
Lancaster L, Cottin V, Ramaswamy M, Wuyts WA, Jenkins RG, Scholand MB, Kreuter M, Valenzuela C, Ryerson CJ, Goldin J, Kim GHJ, Jurek M, Decaris M, Clark A, Turner S, Barnes CN, Achneck HE, Cosgrove GP, Lefebvre c9A, Flaherty KR; PLN-74809-IPF-202 Trial Investigators. Bexotegrast in Patients with Idiopathic Pulmonary Fibrosis: The INTEGRIS-IPF Clinical Trial. Am J Respir Crit Care Med. 2024 Aug 15;210(4):424-434. doi: 10.1164/rccm.202403-0636OC.